General
Fibrous topography of electrospun membrane has been shown to significantly increase the water contact angle of already hydrophobic material compared to its film form. For thermoresponsive hydrophobic material, Poly(N-isopropylacrylamide)/Polystyrene composite nanofiber membrane, the increment in the water contact angle goes from less than 20° to more than 150° in electrospun membrane compared to an increase from 76° to 94° for smooth film at the temperature transitions [Wang et al 2008]. Electrospun core-shell membrane with polycaprolactone as the core and Teflon as the shell demonstrated superhydrophobicity while Teflon film is just hydrophobic [Han et al 2009]. Electrospun gelatin/zein nanofibrous membrane had a hydrophobic surface with water contact angle of 118° but the casted gelatin/zein film had a hydrophilic surface water contact angle of 53.5° [Deng et al 2017].
Size
Typical electrospun nonwoven membrane from hydrophobic materials exhibits water contact angle of more than 100° even for fibers with diameter larger than 7µ. Reduction in fiber diameter generally increases the water contact angle [Ceylan 2006; Cui et al 2008]. Based on the Cassie-Baxter equation, water contact angle is higher for a droplet sitting on cylinder with smaller diameter and on spheres compared to cylinders of comparable radius [Ma et al 2005]. Experimentally, it has been shown that the presence of more beads will result in greater water contact angle [Ma et al 2005; Shao et al 2009] in agreement with the equation. More beads and fibers with smaller dimension will also increase the surface roughness of the material and this would also contribute to greater water contact angle. In an in-depth study on wettability of electrospun membrane, Szewczyk et al (2019) suggested that for smooth electrospun fibers, fiber diameter does not directly influence its membrane wettability, instead, it is membrane roughness and fraction of fiber In the membrane that influence wettability. Fiber diameter is found to be directly proportional to membrane roughness but have no correlation to fraction of fiber. From three groups of electrospun poly(methyl methacrylate) (PMMA) membranes with diameters of 0.34 µm, 1.43 µm and 2.57 µm, the resultant water contact angles are close to 120°.
Surface Roughness
Surface roughness of electrospun fiber can be modified by introducing wrinkles, grooves and pits on it [Miyauchi et al 2006]. Presence of such surface topography is in turn influenced by the choice of solvents, their volatility and their interaction with the polymer. By varying the ratio of tetrahydrofuran (THF) and N,N-dimethyl formamide (DHF) for dissolving polystyrene, Miyauchi et al [2006] was able to electrospin fibers with different surface topography and roughness. In general, nonwoven membrane consisting of smooth surface fibers made out of hydrophobic material is able to give a static water contact angle of more than a hundred degrees but not reaching the superhydrophobicity angle of 150°. Using THF/DMF weight ratios of 2/2 and 1/3 and polystyrene (PS) solution concentration of 30 wt%, fiber with numerous and evenly distributed pits and grooves were electrospun. Subsequently, water contact angle of 158° and 160° respectively were obtained. For the nonwoven membrane with the higher water contact angle, the roll-off angle was 8° thus satisfying the criteria of superhydrophobicity [Miyauchi et al 2006]. Increasing the relative humidity of the electrospinning environment has been shown to introduce pores on the electrospun fiber surface. Liu et al (2018) showed that by electrospinning poly(lactic acid) (PLA) in an environment with increasing humidity, more pores were generated on the electrospun fibers and this led to greater surface roughness. Rising the environmental relative humidity from 40% to 80% increases the membrane porosity from 81% to 92%. Electrospun non-porous PLA membrane has a water contact angle of 121° but electrospun porous PLA membrane at relative humidity of 80% has a water contact angle greater than 150°.
Several studies have found that having beaded fibers generally increases the static water contact angle and it is not uncommon to find beaded electrospun fiber membranes having water contact angle above 150 °. To fabricate beaded electrospun fibers, reducing the concentration is the easiest and well known method. However, reducing electrospinning solution concentration to get more beads may not always yield higher water contact angle. Diaa et al used electrospinning to coat nanofibers, with and without beads, on metal surface to render it superhydrophobic. In their experiment, they showed using both polystyrene (PS) and polymethylmethacrylate (PMMA) fibers, that electrospinning at the lowest concentration did not give the highest water contact angle. There is an optimum solution concentration where the resultant fibers with beads gave the highest water contact angle. Unfortunately, there is no surface roughness values for the fibers produced under those solution concentration.
Electrospun poly(4-methyl-1-pentene) (PFMOP) membrane demonstrated water contact angle above 150° although the roll-off angle is unknown. Scanning electron microscope images showed fibers from beaded to wrinkled ribbon shape at varying electrospun solution concentration [Patel et al 2012]. This may account for the high water contact angle of the membrane.
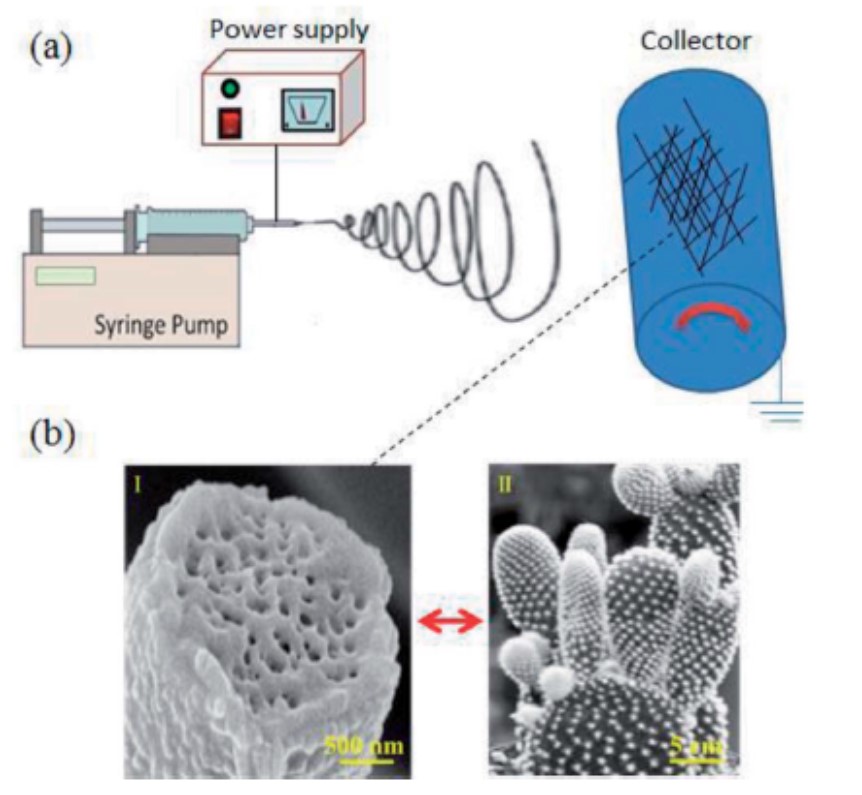
Electrospinning at high humidity was found to create cactus-like surface topography on polyvinylidene fluoride (PVDF) dissolved in a mixture of acetone (ACE) and N,N-dimethylformamide (DMF). Zaarour et al (2018) attributed the formation of the protrusions from the accumulation of acetone vapour trapped beneath the solidified skin of PVDF in high humidity. High humidity and water condensation on the surface of the fiber during eletrospinning causes rapid precipitation of PVDF and forming a skin layer. The accumulated trapped vapour pushes the skin at various points on the fiber, creating protrusions on the surface which were later closed up as water on the surface caused precipitation at the exit points. The escaped vapours would also leave behind pores within the fiber core.
At a lower relative humidity of 2%, smooth surface PVDF fibers were formed and the water contact angle of the electrospun membrane was 81°. At a relative humidity of 62%, surface protrusions were formed on the fibers and the increased roughness of the membrane increases the water contact angle to 156°.
Xue et al (2009) also demonstrated superhydrophobicity from electrospun polyhedral oligomeric silsesquioxanes - polymethylmethacrylate copolymer fibers with water contact angle of more than 160° and sliding angle (contact angle hysteresis) of less than 10 °. Although the fiber diameter was about 2 to 3 µm, they were found to exhibit secondary surface structure and were made out of fibrils with diameter between 50 to 100 nm. The increased surface roughness due to the fibrils running along the length of the fiber probably contributed to its superhydrophobicity.
Another method of introducing surface roughness on electrospun fiber is to use layer-by-layer technique. Ogawa et al [2007] first electrospun cellulose acetate nanofibrous membrane followed by alternate coating with poly(acrylic acid) and TiO2 nanoparticles. The TiO2 nanoparticles form grain-like surface texture on each nanofiber thereby increasing its surface roughness. A final coating of fluoroalkylsilane creates a superhydrophobic membrane with water contact angle of 162° and roll-off angle of 2° for ten bilayers of TiO2/PAA. However, reduced hydrophobicity was observed for greater bi-layers. Without the textured surface, a smooth surface cellulose acetate nanofiber membrane coated with fluoroalkylsilane exhibited a water contact angle of 138°.
Simultaneous electrospinning and electrospraying can be used to disperse nanoparticles onto nanofibers. Su et al (2016) used this technique to disperse silica nanoparticles onto the surface of polyvinylidone fluoride fibers. The resultant hierarchical nanoparticle-microbead-fiber significantly increases the surface roughness of the electrospun membrane. With this combination of hydrophobic material and structural characteristic, a high water contact angle of 163° and low sliding angle of 3° was recorded. Chen et al (2019) created a hierarchically organised structure made out of electrospun PVDF fibers with ZnO protrusions to replicate the micro/nano scale convex papillae and tubular crystalloids structure on the lotus leaf. The surface was further covered with oleic acid to imitate the low surface energy of lotus leaf. Construction of the hierarchically organised structure begins with electrospinning PVDF and ZnO nanoparticles. ZnO arrays were subsequently grown on the surface of the fibers using hydrothermal process followed by coating with oleic acid. The membrane was found to exhibit water contact angle of more than 150° and a roll off angle of 15°. Droplets of water were able to bounced off the surface when dropped from height and the rolling water was demonstrated to pick up dirt hence exhibiting self cleaning property.
Composite
To increase the hydrophobicity of electrospun membrane, a hydrophobic component may be added to it or its surface may be modified. Ding et al [2008] was able to transform zinc oxide nanofibrous film from superhydrophilic (WCA of 0°) to superhydrophobic (WCA of 165° and roll-off angle of 5°) by surface coating with fluoroalkylsilane. Fluoropolymers such as Teflon AF has been electrospun as the sheath of a core-sheath fibrous membrane using polycaprolactone as the core [Han et al 2009]. The composite fibrous membrane demonstrated superhydrophobicity with water contact angle of 158° and roll-off angle of 5°. Hydrophobicity of the membrane is significantly greater than Teflon AF film which has a water contact angle of 120° and 25 ° roll-off angle. Secondary components may also be introduced to increase the surface roughness of the electrospun nanofiber such as through layer-by-layer technique using nanoparticles [Ogawa et al 2007]. Lasprilla-Botero et al (2018) used electrospun polycaprolactone (PCL) fibers and silica (SiO2) microparticles as a coating to increase the hydrophobicity of low-density polyethylene (LDPE) film. In their application, the electrospun PCL fibers worked as a carrier for the electrosprayed silica microparticles. Their tests showed that a longer electrospinning duration for PCL is needed to increase the hydrophobicity of the coated film given the same electrospraying duration of 1h of silica microparticles. A 10 minutes deposition duration for PCL in the LPDE/PCL/SiO2 film gave a water contact angle of 98.8° but a 120 minutes deposition duration for PCL increases the water contact angle to 157.4°and a sliding angle of 8° which makes it superhydrophobic.
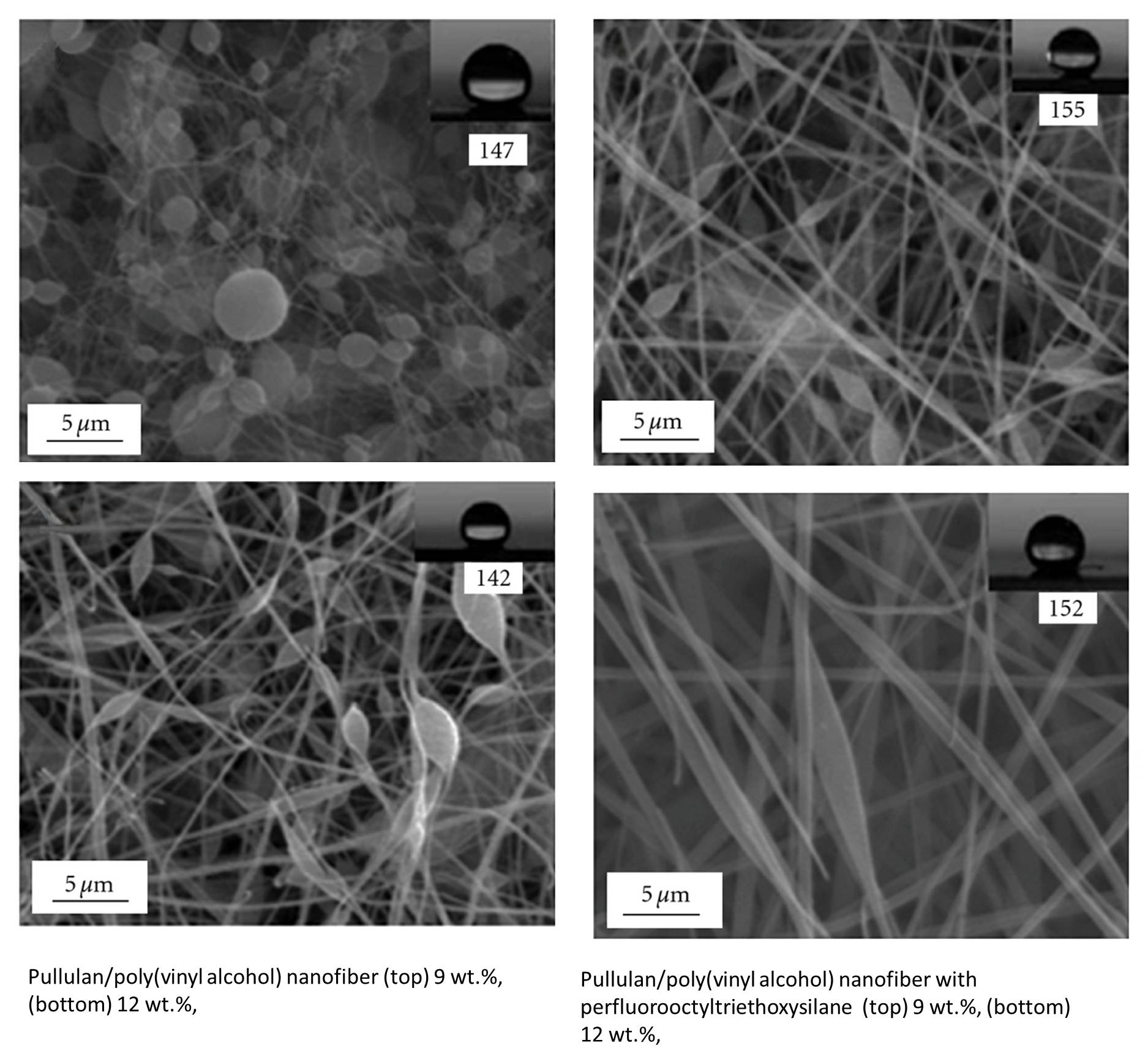
Blending hydrophobic compounds into the solution for electrospinning has also been shown to increase the water contact angle of the resultant electrospun membrane. Karim et al [2011] mixed hydrophobic perfluorooctyltriethoxysilane into a solution of pullulan/poly(vinyl alcohol) before electrospinning to increase the water contact angle of the resultant membrane from 142° to 152°. This is despite pullulan/poly(vinyl alcohol) membrane having beaded fibers while the same material but with perfluorooctyltriethoxysilane added having less beads. Lei et al (2017) used soot nanoparticles to improve the hydrophobicity of electrospun polyvinylidene fluoride (PVDF) fibers. The soot nanoparticles were generated by burning paraffin candles under a metal sheet. Freshly obtained soot particles are known to exhibit superhydrophobicity. Although they are insoluble in PVDF solution, soot nanoparticles can still be well dispersed into the solution due to its viscosity. Observation of the electrospun PVDF/soot nanoparticles under SEM showed uniform dispersion of nanoparticles on the surface of the fibers. Static water contact angle (WCA) tests on the composite gave a high angle of 162° and roll-off angle of less than 5°.
Electrospinning and electrospraying may be used in combination to modify the property of the membrane. Zhang et al (2022) constructed a superhydrophobic membrane by electrospraying polydimethylsiloxane (DP8)/SiO
2 microspheres onto an electrospun polyvinylidene fluoride (PVDF) membrane. Electrospun pure PVDF membrane has a water contact angle of 121.9° which makes it hydrophobic. The addition of electrosprayed DP8 microspheres increases the water contact angle to 159.3°. With electrosprayed DP8/SiO
2 microspheres with 1.5% SiO
2 on the PVDF membrane, the composite membrane has an ultra-high hydrophobic angle of 162.1°. The PVDF/DP8/SiO
2 composite membrane had a distinct hierarchical structure with the microspheres forming an obvious honeycomb structure on the base fiber membrane layer which would have contributed to the surface roughness of the membrane. This coupled with the hydrophobic nature of DP8 and SiO
2 was able to yield the ultra-high hydrophobic angle property.
Material and molecular arrangement
The simplest method of obtaining a superhydrophobic membrane is by electrospinning polymer that is already showing high hydrophobicity. Patel et al (2012) successfully electrospun poly(4-methyl-1-pentene) using a combination of three solvents (cyclohexane, acetone, and N-N-dimethylformamide (DMF)) mixed in a ratio of 80/10/10 by weight heated to 70 °C. The resultant nanofibrous membrane exhibited water contact angle more than 150° and hysteresis angle of less than 10°. Comparison of the hydrophobicity of the membrane with different fiber diameter showed higher hydrophobicity (larger water contact angle and lower hysteresis angle) for smaller fiber diameter. Although electrospinning of poly(4-methyl-1-pentene) without further process optimization or post treatment was able to generate superhydrophobic membrane, other materials may require particular attention to bring their hydrophobic groups close to the surface for the membrane to demonstrate superhydrophobicity.
In the transition from solution to solid form, the arrangement of the polymer chain may be influenced by the processing conditions thus affecting its surface properties. The interaction between solvents and polymer molecules may have an influence on the surface property of the fabricated material. Factors such as group polarity and electronic interaction may drive certain groups within the polymer molecule to the surface and this has been shown to affect the water contact angle of the resultant electrospun membrane. Comparison of the surface groups of electrospun fibrous membrane and solvent cast films revealed migration of hydrophobic methyl group of poly(dl-lactide) to the surface for the former process [Cui et al 2008]. This may contribute to the higher water contact angle on electrospun membrane compared to cast film. A study by Mikaeili et al (2018) suggested that hydrophobicity of conventionally hydrophilic material may increased significantly due to changes in its molecular arrangement during electrospinning. Cellulose acetate (CA) thin film from spin-coating has a water contact angle of 63.67° which is hydrophilic. However, after electrospinning, the CA nanofibrous membrane with nanofiber diameter 350-400 nm, has a contact angle of 154.3°. Their calculation showed that Cassie's model alone is not able to explain such a high water contact angle. Investigation into its molecular structure suggested that concentration of the hydroxyl groups on the electrospun fiber is much lower than spin coated CA thin film. The reduction in hydroxyl groups lowers the surface energy and thus increases its hydrophobicity. It is important to note that while the electrospun CA membrane showed high static water contact angle, the hysteresis of the rolling angle of the membrane is very high too and the droplet does not roll off even when it was held at 90°.
Using poly[(alanino ethyl ester)0.67 (glycino ethyl ester)0.33 phosphazene] (PAGP), Lin et al 2010 showed higher water contact angle when the polymer was electrospun using tetrahydrofuran (119°) as solvent compared with trifluoroethanol (102°) [Lin et al 2010].
Poly(N-isopropylacrylamide) (PNIPAAm)/polystyrene (PS), a thermo-responsive composite electrospun films were also found to exhibit different water contact angle change at the transition temperature when different solvents were used. Although the physical characteristic of the fibrous film is not the same for each solvent, the composite does not show any change in the water contact angle at the transition temperature when dimethyl formamide was used as a solvent (0o) and small water contact angle increment when chloroform (133° to 142°) was used as the solvent. In contrast when tetrahydrofuran was used as the solvent, the water contact angle change from 6° to 152° at the transition temperature of 50°C. Since the difference in physical property of the fibrous film could not have cause such a vast difference in the material surface property, this is likely due to the solvent-polymer interaction that brings about differing molecular arrangements. Wang et al (2014) hypothesized that the polarity value of tetrahydrofuran (THF) which lies between dimethyl formamide and chloroform allows greater free distribution of the PNIPAAm and PS molecules in the THF solution. This allows the PNIPAAm molecules to swell and shrink freely during the transition temperature and alter the water contact angle accordingly. Unfortunately, this has not been verified by surface atomic distribution characterization.
Some materials undergo transformation depending on the storage conditions and this brings about a change in its hydrophobicity. Loccufier et al (2019) showed that their silica nanofibers from sintering of electrospun silica sol gel was hydrophobic right after its formation with a water contact angle of 140°. However, depending on the humidity of the storage condition, residual ethoxy groups due to the incomplete hydrolysis during the production of the sol-gel system prior to electrospinning reacts with the moisture in the air leading to the removal of hydrophobic ethyl groups and forming silanols instead. This changes the membrane from hydrophobic to hydrophilic with contact angle of zero. At 65% relative humidity, the transformation of hydrophobic to hydrophilic occurred after 4 months while at 90% relative humidity, the transformation took 15 days. Poly(2-n-propyl-2-oxazoline) (PnPrOx) is a polymer that exhibits lower critical solution temperature (LCST) behavior where it becomes water soluble below its LCST (~25 °C) and insoluble above it. Electrospun PnPrOx had a surface contact angle of 119° which is much more hydrophobic than its thin film form (surface contact angle of 80°). However, this surface contact angle of the water droplet on the electrospun membrane cannot be maintained as the droplet gets absorbed by the membrane. With lower temperature, the speed of water absorption gets faster. Below its LCST, the membrane is dissolved by the water. A biocompatible cross-linking agent such as tannic acid may be used to maintain the electrospun membrane integrity even at low temperature. With cross-linking, the surface becomes more hydrophilic and the speed of water absorption is increased. The behaviour of water absorption is different with and without cross-linking. Without cross-linking, the water droplet is absorbed through the membrane instead of spreading across its surface. With cross-linking, the water droplet is absorbed across the surface of the membrane.
Pinning state of a water droplet on hydrophobic membrane
As mentioned earlier, electrospun membrane has been shown to enhance the hydrophobicity of hydrophobic material. However, there are instances where despite having surface contact angle of more than 150°, the water droplet seems to stick on the membrane surface instead of rolling off when it is tilted. Wu et al (2009) tested the hydrophobicity of beaded electrospun membrane made of fluorine-containing polyurethane and found that despite having a large water contact angle (WCA) of 159°, it does not demonstrate self-cleaning effect. A 5 microlitre droplet was found to stick on the substate despite been tilted vertically. It is only when a 20 microltire water droplet is used and the membrane tilted at 40° inclination that the water droplet rolls off. Wu et al (2009) attributed this phenomena to the sucking disk effect. A water droplet resting on the nanofiber membrane surface is able to push out the air between the droplet and the nanofiber contact surface thus creating a slight vacuum under the droplet, just like a soft disk on a flat surface. The outside atmospheric pressue will cause the droplet to adhere to the substrate. A larger volume water droplet is able to overcome this slight vacuum as its weight is able to overcome the vacuum under the force of gravity. Presence of fluorine poor region on the fiber surface due to faster vaporization during electrospinning may also contribute to the formation of a complete seal of the cavity below the droplet.
Fiber coverage
The fraction of fiber or fiber coverage in electrospun membrane which is opposite of air volume fraction, was used by Szewczyk et al (2012, 2019) to determine the wettability of the membrane. This value may be determined from two-dimensional (2D) images of the membrane to give an estimate of the air pockets between fibers at the surface of the membrane. The fiber coverage is correlated to the volume of air trapped at the surface of the membrane which is found in the Cassie-Baxter wetting regime for hydrophobicity. Szewczyk et al (2019) investigation using poly(methyl methacrylate) (PMMA) electrospun membrane and film showed that as the fiber coverage reduces, the contact angle increases for water and glycerol. Note that PMMA film assumes a fiber coverage of 100%. A lower fiber coverage increases the air fraction trapped between the fibers and this led to a decrease in surface free energies. For electrospun membrane of poly(lactic-co-glycolic acid) (PLGA), polycarbonate (PC), polycaprolactone (PCL), polystyrene (PS), polyvinylidene fluoride (PVDF) and PMMA, there is an increase in contact angle compared to films made out of the same polymer solution. The exception is polyamide (PA6) where the water contact angle on electrospun membrane is slightly lower than film as water percolates into the fiber networks.
Wenzel model or Cassie-Baxter model
The Wenzel model, and Cassie-Baxter model are the most commonly cited models for explaining the hydrophobicity of a material surface. In the Wenzel model, hydrophobicity is explained by the surface roughness of the material. In the Cassie-Baxter model, hydrophobicity is explained by the air pockets trapped by the water droplet and the surface pores of the material.
Electrospun membrane is generally known to exhibit hydrophobic characteristics. Polyvinyl Butyral (PVB) has a water contact angle of 78° to 83° in film form which is hydrophilic but the same polymer showed water contact angle of 130° to 135° when electrospun which makes them hydrophobic. Applying both models on PVB film and electrospun membrane, Chen et al (2019) found that the film obeys the Wenzel model while electrospun membrane followed the Cassie-Baxter model.
Definition
A surface is considered hydrophobic when the surface contact angle is more than 90° (ASTM D7334) and superhydrophobic when it is more than 150° with roll-off angle/contact angle hysteresis of less than 10° (Wikipedia).
|
Published date: 10 Jan 2014
Last updated: 05 March 2024
▼ Reference
- Ceylan M. Superhydrophobic behavior of electrospun nanofibers with variable additives. Master of Science Thesis, Wichita State University 2006.
-
Chen R, Wan Y, Wu W, Yang C, He J H, Cheng J, Jetter R, Ko F K, Chen Y. A lotus effect-inspired flexible and breathable membrane with hierarchical electrospinning micro/nanofibers and ZnO nanowires. Materials & Design 2019; 162: 246.
Open Access
-
Chen S, Liu G S, He H W, Zhou C F, Yan X, Zhang J C. Physical Structure Induced Hydrophobicity Analyzed from Electrospinning and Coating Polyvinyl Butyral Films. Advances in Condensed Matter Physics 2019: 2019: 6179456, 5 p.
Open Access
-
Cui W, Li X, Zhou S, Weng J. Degradation patterns and surface wettability of electrospun fibrous mats. Polymer Degradation and Stability 2008; 93: 731.
-
Deng L, Kang X, Liu Y, Feng F, Zhang H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocolloids 2017 Article in press
-
Diaa B M, Jaafar H T. Superhydrophobic Nanocomposites Coating Using Electrospinning Technique on Different Materials. International Journal of Applied Engineering Research 2017; 12: 16032. Open Access.
Open Access
-
Ding B, Ogawa T, Kim J, Fujimoto K, Shiratori S. Fabrication of a super-hydrophobic nanofibrous zinc oxide film surface by electrospinning. Thin Solid Films 2008; 516: 2495.
-
Han D, Steckl A J. Superhydrophobic and Oleophobic Fibers by Coaxial Electrospinning. Langmuir 2009; 25: 9454.
-
Karim M R, Islam M S. Thermal Behavior with Mechanical Property of Fluorinated Silane Functionalized Superhydrophobic Pullulan/Poly(vinyl alcohol) Blends by Electrospinning Method. Journal of Nanomaterials 2011; 979458.
Open Access
-
Lasprilla-Botero J, Torres-Giner S, Pardo-Figuerez M, Álvarez-Láinez M, Lagaron J M. Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings 2018; 8(5): 173.
Open Access
-
Lei T, Xiong J, Huang J, Zheng T, Cai X. Facile transformation of soot nanoparticles into nanoporous fibers via single-step electrospinning. AIP Advances 2017; 7: 085212.
Open Access
-
Liu Z, Zhao J, Xing J, Xu L, He J. Humidity-induced porous poly(lactic acid) membrane with enhanced flux for oil-water separation. Adsorption Science & Technology 2019 Article in press.
Open Access
-
Loccufier E, Geltmeyer J, Esquivel D, D'hooge D, D'Buysser K, D'Clerck K. Electrospinning of silica nanofibers without carrier polymer for advanced engineering applications. AUTEX2019, 2019.
Open Access
-
Ma M, Mao Y, Gupta M, Gleason K K, Rutledge G C. Superhydrophobic Fabrics Produced by Electrospinning and Chemical Vapor Deposition. Macromolecules 2005; 38: 9742.
-
Mikaeili F, Gouma P I. Super Water-Repellent Cellulose Acetate Mats. Scientific Reports 2018; 8: 12472.
Open Access
-
Miyauchi Y, Ding B, Shiratori S. Fabrication of a silver-ragwort-leaf-like super-hydrophobic micro/nanoporous fibrous mat surface by electrospinning. Nanotechnology 2006; 17: 5151.
-
Ogawa T, Ding B, Sone Y, Shiratori S. Super-hydrophobic surfaces of layer-by-layer structured film- coated electrospun nanofibrous membranes. Nanotechnology 2007; 18: 165607.
-
Patel S U, Manzo G M, Patel S U, Kulkarni P S, George G C. Permeability of Electrospun Superhydrophobic Nanofiber Mats. Journal of Nanotechnology 2012; 483976.
Open Access
-
Schoolaert E, Cossu L, Becelaere J, Guyse J F R, Tigrine A, Vergaelen M, Hoogenboom R, Clerck K. Nanofibers with a tunable wettability by electrospinning and physical crosslinking of poly(2-n-propyl-2-oxazoline). Materials & Design 2020; 192: 108747.
Open Access
-
Shao L J, Wu J, Xu Z K. Electrospraying/Electrospinning of poly(γ-stearyl-L-glutamate): Formation of surfaces with superhydrophobicity. Chinese Journal of Polymer Science 2009; 27: 115.
-
Stachewicz U, Benett C, Barber A H. Wetting of polyamide film surfaces with electrospun nanofibers. Mater. Res. Soc. Symp. Proc. 2012; 1403: 47
-
Su C, Li Y, Dai Y, Gao F, Tang K, Cao H. Fabrication of three-dimensional superhydrophobic membranes with high porosity via simultaneous electrospraying and electrospinning. Materials Letters 2016; 170: 67.
-
Szewczyk P K, Ura D P, Metwally S, Knapczyk-Korczak J, Gajek M, Marzec M M, Bernasik A, Stachewicz U. Roughness and Fiber Fraction Dominated Wetting of Electrospun Fiber-Based Porous Meshes. Polymers 2019; 11(1): 34.
Open Access
-
Wang N, Zhao Y, Jiang L. Low-Cost, Thermoresponsive Wettability of Surfaces: Poly(N-isopropylacrylamide)/Polystyrene Composite Films Prepared by Electrospinning. Macromol. Rapid Commun. 2008; 29: 485.
-
Wang N, Guo F, Wu J, Zhao Y, Jiang L. Variable Responsive Wettability Films via Electrospinning Induced by Solvents. Journal of Nanomaterials 2014; 817418, 7 pages.
Open Access
-
Wu W, Zhu Q, Qing F, Han C C. Water Repellency on a Fluorine-Containing Polyurethane Surface: Toward Understanding the Surface Self-Cleaning Effect. Langmuir 2009; 25: 17.
-
Xue Y, Wang H, Yu D, Feng L, Dai L, Wang X, Lin T. Superhydrophobic electrospun POSS-PMMA copolymer fibres with highly ordered nanofibrillar and surface structures. Chem. Commun. 2009; 6418.
-
Zaarour B, Zhu L, Huang C, Jin X. Fabrication of a polyvinylidene fluoride cactus-like nanofiber through one-step electrospinning. RSC Adv., 2018, 8, 42353.
Open Access
-
Zhang C, Yang Y, Luo S, Cheng C, Wang S, Liu B. Fabrication of Superhydrophobic Composite Membranes with Honeycomb Porous Structure for Oil/Water Separation. Coatings. 2022; 12(11):1698.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech