The survivability of an implant is dependent on several factors and one of the most important for long term survivability is adequate angiogenesis. It is only with sufficient network of blood vessels within a scaffold that the cells that populate it survive, integrate and return functionality to the tissue or organ. There are several ways in which electrospun fibers may facilitate angiogenesis of an implant. One way is to use electrospun fibers as the implantable scaffold as it has been demonstrated consistently to be a biocompatible structure. Although electrospun fibers may be used as a standalone implant, it is not always the ideal candidate for the targeted tissue regeneration. Alternative use of electrospun fibers may be to complement or modify alternative scaffolds or as carriers for non-active substances.
There are numerous studies showing that electrospun fibers are able to support endothelial cell (EC) adhesion and proliferation. Electrospun fibers have also been routinely loaded with vascular endothelial growth factor (VEGF) and its release have been shown to promote angiogenesis
in vivo [Del Gaudio et al 2013]. Electrospun fibers loaded with VEGF may be used as a scaffold on its own or integrate with other materials for the purpose of releasing the growth factors.
Conventional 3D printing using a melt writing method can easily form 3D structures with large pore sizes but the struts are typically in the tens to hundreds of micrometres diameter. When implanted, host cells may take some time to adhere and integrate with the scaffold. Yang et al (2020) tested the combination of three-dimensional printing and electrospinning in the construction of abdominal wall scaffolds. Synthesized polylactic-co-caprolactone (PLC) copolymer was used in 3D printing while collagen was electrospun onto the printed layers. 3D printing (3DP) and electrospinning (ESP) was carried out alternately to create layers of electrospun fibers sandwiched between 3D-printed layers. Samples of 3DP/ESP biocomposite scaffolds and 3DP scaffolds were tested in a rat full-thickness abdominal wall defect model for biocompatibility and efficiency. Incorporation of host tissue into the scaffold was greater for the biocomposite group compared to the 3DP group in the first 2 few weeks. This is probably due to the presence of electrospun collagen fibers which offer better cell adhesion. At the end of 12 weeks, integration with the host tissue was also stronger for the biocomposite group than 3DP group as evident from higher tensile strength.
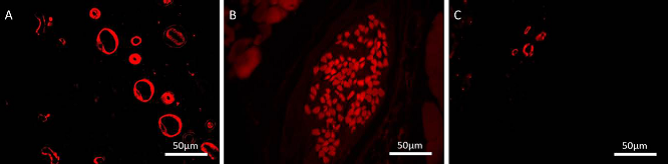
As a scaffold, electrospun fibers have been made into a 3D foam structure and mixed with minced fats for use as tissue fillers. The advantages of using minced fats are that it already contains endothelial cells (EC) amongst others and blood vessel fragments. A study by Panneerselvan et al (2015) showed blood vessels sprouting from the bioartificial scaffold into the surrounding matrigel
in vitro. This shows that the bioartificial scaffold with its heterogeneous cell population has the capability to kick start angiogenesis given the right environment.
Electrospun scaffolds with certain configurations have been shown to promote production of endothelial growth factors by cells cultured on it. iu et al (2023) constructed a scaffold with electrospun polycaprolactone (PCL) nanofibrous yarns forming square grids as the base and electrospun gelatin over it. The PCL nanofibrous yarn contained Indocyanine green (ICG) which is a photothermal dye that is capable of converting the absorbed light energy into thermal energy and welding the fibers. Human adipose stem cells (hADSCs) were cultured on the electrospun scaffold for 24 h and the cell cultured medium was then extracted and tested for its influence on human umbilical vein endothelial cells (HUVECs). hADSCs cultured on the scaffold with the smallest square grid (3 mm length) (SNSG) had the highest viability and proliferation. The conditioned medium from the SNSG group also showed the highest level of endothelial growth factor (EGF). When the conditioned medium was added to HUVECs culture on matrigel, the HUVECs showed greater propensity for tube formation. In an in vivo study using a rat model with full-thickness skin defect of 13 mm in diameter, the group containing hADSCs seeded in SNSG showed the highest CD31 expression in the wound at day 14 compared to other groups including seeded ADSCs without scaffold and SNSG scaffold alone.
Formation of vascular tubules requires the endothelial cells to come together and align in a given direction. Thus, scaffold-directed endothelial cell alignment may be the first step towards encouraging angiogenesis. Studies by Montero et al (2014) and Brown (2012) have shown that aligned fibers were able to guide endothelial cells growth and their formation to functional vessels while random fibers results in scattered endothelial cells. Brown (2012) further studied the effect of aligned fiber diameter on the quantity of in vivo blood vessel formation in rat spinal cord injury. Using polydioxanone aligned fibers of average diameter 1 µm and 2 µm, it was found that the smaller fiber diameter yield more blood vessels.
Another use of electrospun fibers is that it may be used as a sacrificial template for the creation of interconnected channels within a larger bulk matrix. However, since electrospun fibers are typically in the low microns to the hundreds of nanometer diameter, the resultant ultra-small diameter channels may not be suitable for rapid cell migration. In this case, electrospinning may be used with other 3D printing methods to create favorable scaffold for cell migration and to maintain cell viability. The purpose of the channels formed by electrospun fibers is to provide inter-connecting channels and increasing its porosity. Sun et al (2016) demonstrated the usefulness of such a construct using gelatin as the main hydrogel scaffold, printed Pluronic F127 as sacrificial for macro-channels and electrospun PCL as sacrificial for inter-connected micro-channels. For the scaffold with the inter-connected micro-channels from electrospinning, seeded vascular endothelial cells (VEC) showed much better viability compared to scaffolds with only macro-channels.
Published date: 14 February 2017
Last updated: 02 January 2024
▼ Reference
-
Brown D E. Angiogenesis in Response to Varying Fiber Size in an Electrospun Scaffold In Vivo. MSc Thesis. Virginia Commonwealth University 2012.
Open Access
-
Del Gaudio C, Baiguera S, Boieri M, Mazzanti B, Ribatti D, Bianco A, Macchiarini P. Induction of angiogenesis using VEGF releasing genipin-crosslinked electrospun gelatin mats. Biomaterials 2013; 34: 7754.
-
Liu N, Zhou Z, Ning X, Zhang X, Guo Q, Guo M, Wang Y, Wu T. Enhancing the paracrine effects of adipose stem cells using nanofiber-based meshes prepared by light-welding for accelerating wound healing. Materials & Design 2023; 225: 111582.
Open Access
-
Montero R B, Vazquez-Padron R I, Pham S M, D'Ippolito G, Andreopoulos F M. Electrospun Gelatin Constructs with Tunable Fiber Orientation Promote Directed Angiogenesis. Open Journal of Regenerative Medicine 2014; 3: 1.
Open Access
-
Panneerselvan A, Nguyen L T, Su Y, Teo W E, Liao S, Ramakrishna S, Chan C W. Cell viability and angiogenic potential of a bioartificial adipose substitute. J Tissue Eng Regen Med. 2015; 9: 702.
-
Sun Y, Liu Y, Li S, Liu C, Hu Q. Novel Compound-Forming Technology Using Bioprinting and Electrospinning for Patterning a 3D Scaffold Construct with Multiscale Channels. Micromachines 2016; 7: 238.
www.mdpi.com/2072-666X/7/12/238/htm
-
Yang Z, Song Z, Nie X, Guo K, Gu Y. A smart scaffold composed of three-dimensional printing and electrospinning techniques and its application in rat abdominal wall defects. Stem Cell Res Ther 2020; 11: 533.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech