Electrospinning is often carried out at room condition and this allows encapsulation of temperature sensitive materials and organisms. This has allowed researchers to seek potential application using fibers with cells, microbes and bacteria encapsulated within the fibers. A potential application is the introduction of microbes to rebuild microbiota in the body. Since living organisms are sensitive to its environment, the electrospinning process and solution composition needs to be studied and optimized to ensure the viability of the encapsulated organism.
Several studies have demonstrated the electrospinning of fibers with living cells encapsulated and that the cells are able to survive the electrospinning process although the viability of the cells varies from less than 20% [Zanatta et al 2012] to more than 60% [Townsend-Nicholson et al 2006]. The specific methods of electrospinning cells probably accounts for the difference in cell viability. Direct blending of cells in polymer solution and electrospinning will subject the cells to more shearing force as compared to a core-shell setup where the cells are surrounded in less viscous media at the core of the setup. The selection and composition of the solution which contain the cells have a significant impact on its viability following electrospinning. Using a core-shell electrospinning setup, Ang et al (2014) found that the addition of fetal bovine serum (FBS) to the core solution containing the cells creates a more isotonic environment which improves cell viability. In the polyethylene oxide (PEO) solution with FBS, cell viability of more than 95% was registered in the first 2 hours. However with fibers electrospun from PEO solution alone, the number of viable cells was reduced to less than 3% over the same period.
Contrary to the report by Ang et al (2014), Xu et al (2022) was able to achieve 90.15% survival rate immediately after electrospinning with bone marrow-derived stem cells (BMSCs) added to polyvinyl alcohol (PVA) solution prepared using phosphate-buffered saline (PBS). It will be interesting to determine the solution properties that affect the viability of the cells. Both Ang et al (2014) and Xu et al (2022) used water soluble polymers in aqueous media for their cell electrospinning hence there is no toxicity effect. Viscosity of the solution may be a possible parameter that affects cell viability due to the transmission of shear forces during electrospinning.
Jayasinghe et al (2015) studied the viability cells from core-shell cell electrospinning by examining the binding of Annexin V and/or propidium iodide (PI) to the luciferase-transduced murine (IC-21-Luc) macrophages. This allows them to classify the cell population into live, early apoptotic, late apoptotic and dead. Their study showed no apparent differences in the viability of electrospun cells compared to cell culture controls over a 72 h period with cell viability above 90%. Collagen hydrogel for cell electrospinning was modified by blending concentrated laminin to increase their viscosity [Jayashinghe et al 2015].
Yang et al (2022) also used coaxial electrospinning to produce fibers with cells. The human bone mesenchymal stem cells (hBMSCs) used in their study was loaded onto the shell of their poly (3-hydroxybutyrate-co-4-hydroxybutyrate) (PHB) core and poly (vinyl alcohol) (PVA) shell fiber instead of the core. PVA was used as the shell material as it is water soluble and the cell culture media containing hBMSCs was mixed with the PVA solution before electrospinning. Although the core material, PHB, was dissolved in CH2CL2, the solvent does not seem to have a significant impact on the viability of the cells. The cell survival rate at 3 days was found to be 97% which is the same as the control where cells are seeded onto electrospun PHB/PVA fibers.
With cells encapsulated in a medium, methods such as electrospinning and 3D cell printing may be used to construct scaffolds containing cells. In electrospinning, the process has the benefit of aligning the cells along the fiber axis which may not be easily achieved using other methods.
Yeo et al (2023) prepared a cell-laden bioink containing alginate and polyethylene oxide (PEO) (A2P3) in water. Mesenchymal stem cells (MSCs) and smooth muscle cells (SMCs) were added to the A2P3 aqueous solution prior to electrospinning and 3D cell printing (CP). Cell viability after 7 days of culture was found to be more than 90% for both CP scaffold and electrospun scaffold. The cell-printed scaffold had a plain bulk structure and the cells had a round shape while cells in the electrospun scaffold showed elongation morphology along the length of the fiber. Cells on electrospun scaffold showed higher F-actin aspect ratios and orientation factor and the SMCs showed greater expressions of fibronectin, collagen I and collagen (IV). Therefore the ability of electrospinning to produce finer structure compared to cell printing was able to produce a scaffold more conducive for cell expression.
Microbes and bacteria have also been encapsulated within fibers using electrospinning. Salalha et al (2006) tested the viability of a range of bacteria and virus following electrospinning. The tested bacteria were Escherichia coli and Staphylococcus albus and the virus were bacteriophages T7, T4 and λ. Viability of the bacteria were found to be better with 19% for E. coli and 100% for S. albus while those of the virus were poor with less than 10% viability. These differences were attributed to the presence of cell wall for the bacteria which are better able to withstand the shear force during electrospinning. Salalha et al (2006) hypothesized that it is the rapid vaporization of the solvent during electrospinning resulted in the relatively poorer viability of E. coli compared to S. albus. By culturing E. coli in 5% glycerol, the viability of E. coli was significantly raised to 48%. The presence of glycerol in the E. coli probably protects the cell from rapid dehydration during electrospinning. Diep et al (2021) also investigated the viability of E. coli after electrospinning in a polymer carrier. For the purpose of developing an edible probiotic delivery system, the material for encapsulating the bacteria was a mixture of biopolymer alginate (SA), the carrier polymer poly(ethylene oxide) (PEO), and the FDA approved surfactant polysorbate 80 (PS80). By checking the excess precursor solution that fell from the needle tip during electrospinning, they found that there is no loss in bacteria viability after being subjected to the effect of the electric field. While the electrospun SA/PEO/PS80 fibers contain viable E. coli, their study did not calculate the percentage loss of bacterial viability although viable concentration of bacteria in the electrospun mat was found to be 2.74 × 105 CFU g-1. Although the bacteria may not be affected by the electric field when they are surrounded by the polymer matrix or solution, stretching and dehydration may have a detrimental effect on the bacteria viability [Salalha et al 2006].
Shin et al (2014) fabricated nanofibrous poly(lactic-coglycolic acid) (PLGA) scaffold containing cell-adhesive RGD peptide-displaying M13 bacteriophage. The PLGA nanofibrous scaffold containing the M13 bacteriophage was able to show significant enhancement in proliferation and attachment of fibroblasts compared to the control.
Early work by Salalha et al (2006) suggest that a stabilizing excipient may be used to improve the viability of microbes encapsulated in electrospun fibers.
Lopez-Rubio et al (2009) tested the viability of Bifidobacterium animalis subsp. lactis Bb12 when encapsulated in polyvinyl alcohol (PVA) in a core-shell setup. B animalis Bb12 was suspended in skimmed milk and encapsulated at the core of the fiber. Comparing the viability of B animalis Bb12 encapsulated in the fiber with non-encapsulated B animalis Bb12 , viability of the encapsulated B animalis Bb12 was significantly higher when both were stored at 4 °C for 130 days. When stored at room temperature and - 20 °C, both showed reduced viability over time.
Stabilizing excipients may work in several ways to maintain viability of encapsulated microbes. Excipients may afford protection due to their amorphous state by restricting molecular movement in their glassy matrix which may damage the microbe. Other excipients may be prebiotics which may help the maintenance of the microbes. Hirsch et al (2021) tested the viability of Lactobacillus paracasei blended in polyvinyl alcohol (PVA)/polyethylene oxide(PEO) solution and stabilizing excipient (glucose, lactose, mannitol, saccharose, trehalose, inulin) and electrospinning. Saccharose, trehalose and skim milk were found to be the most effective excipients and showed similar or better results compared to other drying techniques. L. paracasei dried using electrospinning and skim milk as excipient has a survival rate of 85% compared to just 32% using spray drying using skim milk. Lower temperature was also found to enhance storage survival rate. The viability of the bacteria drops to zero in 7 to 120 days when stored at 25 °C. However, the bacteria remains viable for 1 year of storage at 7 °C and -20 °C after which storage at -20 °C showed better survival rate. The lesser viability at higher storage temperature has been attributed to the movement of molecules at higher temperature which may damage cell walls.
A potential application is in the delivery of microbes and bacteria to specific organs for re-establishment of a healthy population of microbiota. Nagy et al (2014) investigated the use of water soluble polymers (polyvinyl alcohol and polyvinylpyrrolidone) for encapsulation and delivery of Lactobacillus acidophilus bacteria. The survival rate of the bacteria was found to be between 34% and 68%. This electrospun membrane has been developed for potential treatment of bacterial vaginosis. In which case, the water soluble polymer will release its bacteria loads upon application.
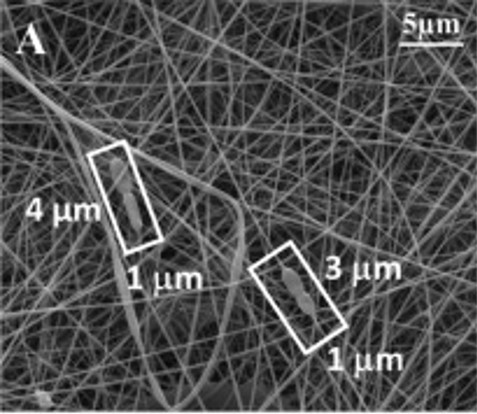
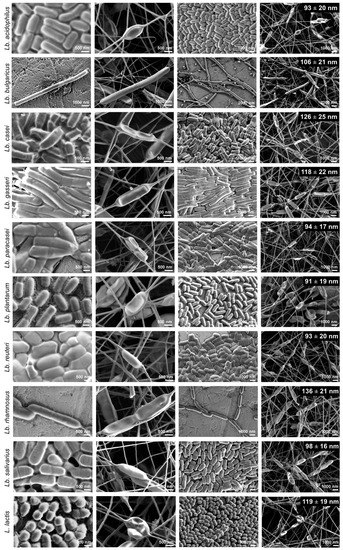
Zupancic et al (2019) studied the effects of electrospinning on the viability of ten species of lactic acid bacteria in poly(ethylene oxide) (PEO) nanofibers. While all the bacterial species are viable following encapsulation in the electrospun nanofibers, their survivability varies. Five species, Lactobacillus (Lb.) acidophilus, Lb. gasseri, Lb. reuteri, Lb. salivarius, and L. lactis showed high viability with survival rate of about 90% and Lb. delbrueckii ssp. bulgaricus was the worst with survival rate of about 50%. Lb. casei, Lb. paracasei, Lb. plantarum, and Lb. rhamnosus showed survival rate in between. In this study, hydrophilic bacteria showed lower survival rate than hydrophobic bacteria. The fact that electrospinning of PEO with the bacteria is done in aqueous media may result in greater shear force on hydrophilic bacteria compared to hydrophobic bacteria. While there are no general correlation found between bacteria size and bacteria viability, the low viability of Lb. delbrueckii ssp. Bulgaricus may be related to its size as it has by far the longest cells.
Stojanov et al (2024) investigated the potential use of electrospun fibers as a platform for delivery of vaginal lactobacilli. Modified lactobacilli (Lactobacillus crispatus, Lactobacillus gasseri and Lactobacillus jensenii) with fluorescent (mCherry and GFP) and luminescent (NanoLuc luciferase) proteins were loaded into polyethylene oxide (PEO) solution for electrospinning into fibers. The release of the bacteria can be monitored through the fluorescence signal and it was shown that about 20 - 60% of the bacteria were released in the first 10 min of incubation and all the bacteria were released in 30 - 45 min. Viability of the fluorescent protein-labelled bacteria after electrospinning to form fibers differed with the most decreased shown by L. crispatus and less decreased by L. gasseri and L. jensenii. Of these three bacteria, L. gasseri and L. jensenii showed high hydrophobicity at 95.8% and 87.3% respectively, as determined by extracting them with n-hexadecan. L. crispatus showed much lower hydrophobicity at 21.4%. The effect of bacteria hydrophobicity on its viability is consistent with the results from
Zupancic et al (2019) where more hydrophilic bacteria may suffer from greater shear force when electrospinning in an aqueous media resulting in reduced viability.
Another targeted organ for re-introduction of beneficial microbes is the gastro-intestinal tract. Liu et al (2016) used an aqueous solution containing two edible polysaccharides, pectin (PEC) and pullulan (PUL) for encapsulation of probiotic bacteria Lactobacillus rhamnosus GG (LGG). The electrospun PEC/PUL fibers containing the bacteria were cross-linked by soaking in 5% CaCl2 solution. 90% of the bacteria were found to remain viable after electrospinning and cross-linking which demonstrates the potential use of edible polysaccharides as bacteria carriers.
To further improve the survivability of probiotics, Akkurt et al (2022) blended aqueous calcium caseinate (CaCAS) and sodium caseinate (NaCAS) with pullulan (PUL) aqueous solutions for electrospinning into fibers. Lactobacillus rhamnosus GG (LGG) was added into the solution just before electrospinning. The resultant electrospun fibers CAS-PUL-LGG had a mean diameter of 212 nm while NaCAS-PUL-LGG fibers had a mean diameter of 286 nm. LGG can be seen as elongated beads on part of the fibers. Viability of the LGG recovered from the nanofibrous mats after electrospinning showed no loss in the bacteria. The presence of milk milk proteins such as caseins may aid the survival of encapsulated LGG within the fibers and may potentially improve the survival of the probiotic if the CAS-PUL-LGG fiber mats were taken orally.
However, further research needs to be carried out to determine the viability of the bacteria in storage.
Vass et al (2020) investigated the use of water soluble hydroxypropyl-beta-cyclodextrin (HP-β-CD) as the carrier polymer for Clostridium butyricum which is a anaerobic spore-forming bacteria normally found in the human gastrointestinal tract. After electrospinning of HP-β-CD loaded with C. butyricum spores or cells, the loaded fibers were grinded into powdered form for compression into pills. These processes did not lead to a reduction in the bacteria viability. Electrospun sporulated C. butyricum was also found to remain viable after 1 year of aerobic storage at ambient temperature. Sporulated C. butyricum contained 30% bacteria spores and 70% vegetative cells. For electrospun vegetative cells, 1 year of aerobic storage at ambient temperature renders them unviable. Therefore, electrospinning is a promising formulation technology for microbiome delivery applications.
In the delivery of live probiotics to the gastrointestinal tract, just as important is that the microbe remains viable after passing through the acidic condition of the stomach. Diep et al (2024) seeks to address this by encapsulating probiotic Lactococcus lactis via coaxial electrospinning into alginate-based nanofibers containing the antacid calcium carbonate. The core-shell fibers were made of poly(ethylene oxide) (PEO), alginate, surfactant polysorbate 80 (PS80) and calcium carbonate (CaCO3) for the shell. The core solution contained alginate and L. lactis. Using a gastrointestinal tract (GIT) model, the core-shell fibers containing the L. lactis were first incubated in an acidic solution mimicking the "stomach" followed by incubating in a solution mimicking the "intestines". In a control core-shell fibers without antacid, no viable bacteria was detected in the stomach phase and intestinal phase. With the fibers containing antacid, no bacteria was found in the stomach phase but viable bacteria can be found in the intestinal phase. This is due to alginate being stable in an acidic environment but swells in higher pH thereby releasing the bacteria. However, in an acidic environment, protons can penetrate the alginate and deactivate the bacteria encapsulated within. Therefore, antacid is necessary to shield the bacteria in the stomach phase.
For utilization of microbes in biotechnology, encapsulation of microbes in fibers will ensure retention of the microbes in the treatment area. However, this is not sufficient as the microbe must be able to interact with the environment despite been surrounded by the matrix material. Tong et al (2013) tested the functionality of E. coli bacteria encapsulated in electrospun silica/polyvinyl alcohol (PVA) fiber with free E. coli. From the degradation of atrazine, they found that the encapsulation did not significantly impede the activity of E. coli. Further the formation of silica/PVA using silica precursor results in a water-insoluble fiber matrix. Rendering a water-soluble material insoluble is important for practical application of microbe-encapsulated fibers in cases where the material is not designed to release the microbes. Liu et al (2009) used water-soluble Pluronic F127 dimethacrylate (FDMA) and polyethylene oxide (PEO) to mix with Pseudomonas fluorescens, Zymomonas mobilis, and E. coli for electrospinning. To cross-link the fibers to render the fibers water insoluble, glycerol was used as the cross-linking medium as it has low toxicity to microorganism. A redox system consisting of ammonium persulfate (APS), ascorbic acid (AsA), and ferrous sulfate was added to the solution of glycerol and water for cross-linking of the fibers. The fibrous structure was retained after removal of PEO. Z mobilis was found to remain viable after storage at 4 °C for up to 7 days although the percentage of bacteria that remained viable was reduced to 23%. The metabolic activity of Z mobilis was not affected by the electrospinning process. Zamel et al (2019) was able to demonstrate the biodegradation of methylene blue (MB) dye with Bacillus paramycoides encapsulated within electrospun cellulose acetate (CA)/poly(ethylene oxide) (PEO) nanofibrous membrane. To ensure viability of the bacteria, dimethyl sulfoxide (DMSO) was selected as the solvent which is known to be safe on bacterial cells. In water, CA/PEO nanofibers swell and this allows the dye to be adsorbed and the reductases enzymes which are produced and secreted by the bacteria to be released into the surrounding media for biodegradation of MB. MB removal by free bacteria and bacteria-immobilized CA/PEO nanofibrous membrane was similar after 48 h at 89.13 and 87.39% respectively. This showed that encapsulation of the bacteria in the electrospun CA/PEO fiber matrix does not reduce its MB removal efficiency. Letnik et al (2015) used core-shell electrospinning to encapsulate yeasts in a non-biodegradable polymer for treatment of polluted water. To expose the yeasts, Candida tropicalis, to the polluted water, they mixed water soluble polyethylene glycol (PEG) with the non-water soluble polyvinylidene fluoride-co-hexafluoropropylene as the shell material such that PEG will leach out and form pores in the outer shell. The bioactive membrane was able to biodegrade 60% of phenols in contaminated water sample in 72 hours. Although this is much slower than unembedded yeasts which biodegrades 100% of the phenols in 24 hours, the bioactive membrane can be reused and the yeasts are not released into the environment.
Encapsulation of microbes in electrospun nanofibers is also useful in agricultural applications. Damasceno et al (2013) used electrospun polyvinyl alcohol (PVA) nanofibers to encapsulate rhizobia, an economically interesting bacterium found in legumes. The PVA matrix potentially protects the rhizobia from environmental stress such as temperature and dehydration. Comparing the viability of rhizobia encapsulated in PVA nanofiber and the negative control (unprotected rhizobia), significantly more rhizobia remains viability after 48 h of storage for those encapsulated in PVA nanofiber. Study was also carried out to determine whether the encapsulation of the bacteria in PVA nanofiber has a negative impact on the number of nodules present in soybean when applied. There is no significant difference in the number of nodules formed between rhizobia encapsulated in PVA nanofiber and the positive control (unprotected rhizobia) over a 30 days period. Therefore, encapsulation of rhizobia in PVA nanofibers is a viable method for storage and delivery of the bacteria.
The relative ease of encapsulating bacteria has seen its use in the construction of Single Chamber Microbial Fuel Cells (SCMFCs). Massaglia et al (2021) tested the feasibility of using electrospinning to encapsulate mixed culture of bacteria from a seawater sediment to form a nanofiber-based bio-composite (bio-NFs) and depositing them on an anode. Polyethylene oxide (PEO) was selected as the carrier polymer due to its water solubility. Encapsulation of the bacteria is by blending with PEO solution. When the electrospun bacteria/PEO fibers were immersed into electrolyte and PBS, the bacteria was found to be viable although the study did not indicate the percentage of viability. By electrospinning bacteria/PEO onto a carbon paper, formation of the biofilm is accelerated due to the proximity of the encapsulated bacteria to the carbon paper surface. After 1 month of acclimation in electrolyte, the bio-NFs anode was able to show a current density that doubles that of the negative controls which were PEO nanofiber and carbon paper without bacteria.
Apart from encapsulating the bacteria within the electrospun fiber matrix, another method is to immobilize the bacteria on the surface of the electrospun membrane through adhesion. Páez-Vélez et al (2020) constructed an electrospun polycaprolactone (PCL) membrane (fiber diameter 3.5 µm) with Lysinibacillus sphaericus immobilized on its surface for the purpose of capturing gold from industrial wastewaters. Immobilisation of L. sphaericus is by dipping the electrospun PCL membrane into the bacteria suspension. Due to the fibrous architecture, L. sphaericus was able to adhere well on the membrane. Sonication and repeated washing was used to remove any non-Immobilized bacteria. The resultant biocomposite was shown to be able to remove 93% of gold ions in the aqueous medium after 120 h while neat PCL was only able to remove 57%. This showed that the presence of L. sphaericus on the electrospun fibers contributed significantly to the removal of gold from the water.
Published date: 21 July 2015
Last updated: 16 December 2025
▼ Reference
-
Akkurt S, Renye J, Tomasula PM. Encapsulation of Lactobacillus rhamnosus GG in edible electrospun mats from calcium and sodium caseinates with pullulan blends. JDS Communications 2022; 3: 381.
Open Access
-
Ang H Y, Irvine S A, Avrahami R, Sarig U, Bronshtein T, Zussman E, Boey F Y C, Machluf M, Venkatraman. Characterization of a bioactive fiber scaffold with entrapped HUVECs in coaxial electrospun core-shell fiber. Biomatter 2014; 4: e28238.
Open Access
-
Damasceno R, Roggia I, Pereira C, Sa E. Rhizobia survival in seeds coated with polyvinyl alcohol (PVA) electrospun nanofibres. Can. J. Microbiol. 2013; 716.
-
Diep E, Schiffman J D. Encapsulating bacteria in alginate-based electrospun nanofibers. Biomater. Sci. 2021; 9: 4364.
Open Access
-
Diep E, Schiffman J D. Targeted release of live probiotics from alginate-based nanofibers in a simulated gastrointestinal tract. RSC Appl. Polym. 2024; 2: 719.
https://pubs.rsc.org/en/content/articlelanding/2024/lp/d4lp00023d Open Access
-
Hirsch E, Pantea E, Vass P, Domján J, Molnár M, Suhajda A, Andersen S K, Vigh T, Verreck G, Marosi G J, Nagy Z K. Probiotic bacteria stabilized in orally dissolving nanofibers prepared by high-speed electrospinning. Food and Bioproducts Processing 2021; 128: 84.
Open Access
-
Jayashinghe S N, Auguste J, Scotton C J. Platform Technologies for Directly Reconstructing 3D Living Biomaterials. Advanced Materials 2015 Article in Press.
Open Access
-
Letnik I, Avrahami R, Rokem J S, Greiner A, Zussman E, Greenblatt C. Living Composites of Electrospun Yeast Cells for Bioremediation and Ethanol Production. Biomacromolecules 2015 Article in press.
-
Liu Y, Rafailovich M H, Malal R, Cohn D, Chidambaram D. Engineering of bio-hybrid materials by electrospinning polymer-microbe fibers. PNAS 2009; 106: 14201.
Open Access
-
Liu S C, Li R, Tomasula P M, Sousa A M M, Liu L. Electrospun Food-Grade Ultrafine Fibers from Pectin and Pullulan Blends. Food and Nutrition Sciences 2016; 7: 636.
Open Access
-
Lopez-Rubio A, Sanchez E, Sanz Y, Lagaron J M. Encapsulation of Living Bifidobacteria in Ultrathin PVOH Electrospun Fibers. Biomacromolecules 2009; 10: 2823.
-
Massaglia G, Sacco A, Chiodoni A, Pirri CF, Quaglio M. Living Bacteria Directly Embedded into Electrospun Nanofibers: Design of New Anode for Bio-Electrochemical Systems. Nanomaterials. 2021; 11(11):3088.
Open Access
-
Nagy Z K, Wagner I, Suhajda A, Tobak T, Harasztos A H, Vigh T, Soti P L, Pataki H, Molnar K, Marosi G. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. eXPRESS Polymer Letters 2014; 18: 352.
Open Access
-
Páez-Vélez C, Castro-Mayorga J L, Dussán J. Effective Gold Biosorption by Electrospun and Electrosprayed Bio-Composites with Immobilized Lysinibacillus sphaericus CBAM5. Nanomaterials (Basel) 2020; 10: 408.
Open Access
-
Salalha W, Kuhn J, Dror Y, Zussman E. Encapsulation of bacteria and viruses in electrospun nanofibres. Nanotechnology 2006; 17: 4675.
-
Shin Y C, Lee J H, Jin L H, Kim M J, Oh J W, Kim T W, Han D W. Cell-adhesive RGD peptide-displaying M13 bacteriophage/PLGA nanofiber matrices for growth of fibroblasts. Biomaterials Research 2014; 18: 14.
Open Access
-
Stojanov S, Plavec T V, Zupancic S, Berlec A. Modified vaginal lactobacilli expressing fluorescent and luminescent proteins for more effective monitoring of their release from nanofibers, safety and cell adhesion. Microb Cell Fact 2024; 23: 333.
https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-024-02612-w Open Access
-
Tong H W, Mutlu B R, Wackett L P, Aksan A. Silica/PVA biocatalytic nanofibers. Materials Letters 2013; 111: 234.
-
Townsend-Nicholoson A and Jayasinghe S N. Cell Electrospinning: a Unique Biotechnique for Encapsulating Living Organisms for Generating Active Biological Microthreads/Scaffolds. Biomacromolecules 2006; 7: 3364.
-
Vass P, Pantea E, Domokos A.Hirsch E, Domján J, Németh A, Molnár M, Fehér C, Anderson S K, Vigh T, Verreck G, Csontos I, Marosi G, Nagy Z K. Electrospun Solid Formulation of Anaerobic Gut Microbiome Bacteria. AAPS PharmSciTech 2020; 21: 214.
Open Access
-
Xu S, Lu T, Yang L, Luo S, Wang Z, Ye C. In situ cell electrospun using a portable handheld electrospinning apparatus for the repair of wound healing in rats. International Wound Journal 2022 Early view.
Open Access
-
Yang L, Zhao Y, Cui D, Liu Y, Zou Q, Xu S, Luo S, Ye C. Coaxial bioelectrospinning of P34HB/PVA microfibers biomimetic scaffolds with simultaneity cell-laden for improving bone regeneration. Materials & Design 2022; 213: 110349.
Open Access
-
Yeo M, Yoon J W, Park G T, Shin S C, Song Y C, Cheon Y I, Lee B J, Kim G H, Kim J H. Esophageal wound healing by aligned smooth muscle cell-laden nanofibrous patch. Materials Today Bio 2023; 19: 100564.
Open Access
-
Zamel D, Hassanin A H, Ellethy R , Singer G, Abdelmoneim A. Novel Bacteria-Immobilized Cellulose Acetate/Poly(ethylene oxide) Nanofibrous Membrane for Wastewater Treatment. Sci Rep 2019; 9: 18994.
Open Access
-
Zanatta G, Steffens D, Braghirolli D I, Fernandes R A, Netto C A, Pranke P. Viability of mesenchymal stem cells during electrospinning. Brazilian Journal of Medical and Biological Research 2012; 45: 125.
-
Zupancic S, Skriec K, Kocbek P, Kristl J, Berlec A. Effects of Electrospinning on the Viability of Ten Species of Lactic Acid Bacteria in Poly(Ethylene Oxide) Nanofibers. Pharmaceutics 2019; 11(9): 483.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech