TiO2 is one of the most widely investigated inorganic oxide produced through electrospinning due to its properties and relative ease of production using precursors. TiO2, rutile and anatase phases are the most commonly encountered and manufactured depending on the applications. The former is mainly for coating and cosmetics purposes due to its high refractive index and UV absorption cross-section and the latter is for catalysis as it is chemically and optically active. A simple method directing the phase formation and transformation is by controlling the calcination temperature. In electrospun TiO2, calcination temperature of less than 400°C produces mainly anatase phase while at temperature more than 600°C, rutile phase dominates [Song et al 2013].
Li et al (2012) showed that with TiO2, calcination of electrospun polyvinyl pyrrolidone/tetrabutyl titanate (PVP/TBT) at temperature of 500°C, 600°C and 700°C for 3 hours gave rise to increasing grain size. This results in a drop in its specific surface area which in turn reduces its photocatalytic activity.
While it is widely accepted that the anatase phase is what gives TiO2 its photocatalytic property, Li et al (2022) suggest that a small amount of rutile phase in a majority anatase phase will give the electrospun TiO2 fiber the highest photocatalytic performance. In their research, the interfacial temperature between anatase and rutile phase occurs at 700 °C instead of 600 °C reported by others. While it is mainly anatase phase when annealed at 700 °C, a small amount of rutile phase can be found. Li et al (2022) hypothesized that the two phases in close proximity to each other forming an anatase-rutile heterojunction resulted in the highest photocatalytic action compared to pure anatase phase TiO2 from lower annealing temperature. Since anatase and rutile phase occupy different bands, the electron generated from the anatase conduction band (CB) would migrate to the rutile phase CB and the corresponding hole from the valence band (VB) of rutile phase to the VB of anatase phase. This improves the separation efficiency of the photogenerated electron-hole pair and reduces its recombination.
Mesoporous structure has a higher surface area compared to solid fibers and this makes it ideal for applications such as sensors, photocatalytic degradation, energy storage and energy conversion. Electrospinning and sintering of some TiO2 precursors are able to form mesoporous fibers, however, electrospinning of PVP/tetrabutyl orthotitanate was shown to give rise to nonporous fibers after sintering. With the addition of P123 as a structure directing agent into the solution for electrospinning, mesoporous TiO2 fibers can be produced after sintering [Wang et al 2012].
Using electrospinning, TiO2 doped with other salt may be produced. These salts may be added with the TiO2 precursor solution for electrospinning. Doped TiO2 has been found to be very useful in improving its sensor applications. Qi et al (2008) showed that KCL-doped TiO2 nanofibers demonstrated impedance variance of more than four orders of magnitude in the range of 11% to 95% relative humidity over pure TiO2 nanofibers. Li et al (2008) also reported improved impedance variance when TiO2 nanofibers were doped with up to 30% LiCl.
Many applications of TiO2 are based on its photo-generated electron-hole pair where the hole in the valence band of TiO2 participated in further reaction. However, rapid recombination of the electron-hole pair reduces the activity of the TiO2. A common strategy is to introduce an alternative material to transfer the photo-generated electron away so that the holes are given sufficient time for further reactions. Koozekonan et al (2021) tested the UV protection ability of electrospun polyacrylonitrile (PAN) loaded with multi-walled carbon nanotubes (MWCNT) and TiO2 nanoparticles and both. Electrospun PAN fibers alone do not offer UV protection and 1% loading of either MWCNT or TiO2 nanoparticles would offer good protection. However, to achieve excellent protection, more than 10% loading of either MWCNT or TiO2 nanoparticles needs to be added. Higher concentration of TiO2 needed to offer excellent UV protection is probably due to recombination of electrons and hole pairs. When MWCNT and TiO2 are added together for electrospinning in PAN, the resultant composite nanofibers require only 1% of the additives to achieve excellent UV protection. MWCNT, being conductive, was able to transfer the photo-generated electron from the TiO2 away and prevented immediate recombination of the electron-hole pair. This leaves behind the hole in the valence band of the TiO2 for reaction.
The introduction of oxygen vacancies in electrospun derived TiO2 nanofibers through H2 plasma treatment has been shown to improve photocatalytic performance. Li et al (2022) showed a 30% improvement in photocatalytic degradation of methyl orange solution in plasma-treated TiO2 nanofibers compared to the same nanofibers without plasma treatment. With greater number of oxygen vacancies, more electrons are trapped. This facilitates the separation of photogenerated electron-hole pairs. The oxygen vacancies may participate in the photocatalytic degradation by converting O2 to reactive O2-.
Hydrothermal post treatment has been used to create inorganic composite fibrous networks with a functional layer on the surface. Depending on the treatment process and material, various secondary structures have been formed on the underlying electrospinning derived inorganic fibers. Zhang et al (2019) used hydrothermal post-treatment to grow NiO Nanosheets@ on TiO2 nanofibers prepared by electrospinning. Construction of the NiO Nanosheets@ is by dipping the TiO2 nanofibers sheet in NiSO4·6H2O and hexamethylenetetramine (HMT) followed by sintering.
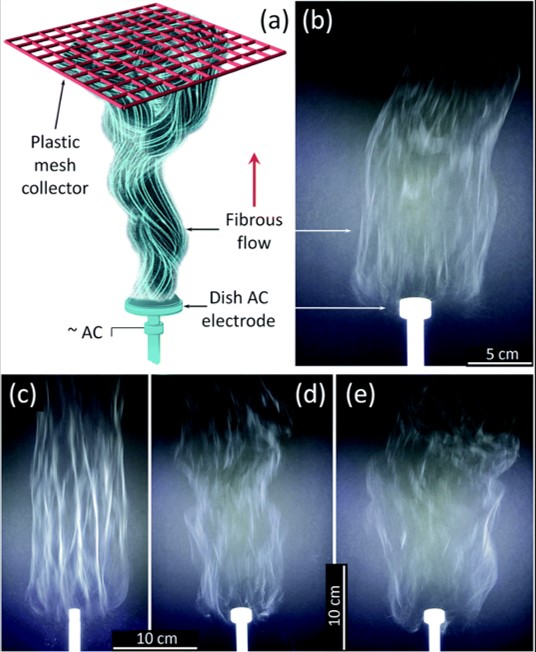
Inorganic nanofibers such as TiO2 require sintering of its electrospun precursor material. Since there will be further weight loss from the precursor material form, feasible commercial application of such inorganic nanofibers would require the development of mass production methods. Nealy et al (2020) used an alternating current (AC) on a free surface electrospinning setup to electrospin a solution of titanium(IV) n-butoxide (Ti(OBu)4)/ polyvinylpyrrolidone (PVP), with alternating current (AC)-voltages up to 40 kV rms at 60 Hz was used. The polymer solution was fed into a shallow dish-like electrode where the AC high voltage was applied. The collector was a PTFE plastic mesh placed about 50 cm above the electrode. Dish-like electrodes with diameters from 10 to 25 mm were tested. With smaller diameter electrodes, an increase of fiber bundling was observed which is not desirable. The feed-rate of the solution was adjusted to support a stable generation of fibers. Assuming the precursor fibers were converted to TiO2, the production rate of TiO2 nanofiber from one electrode is about 5.2 gh-1. A 5 - 7 cm thick fluffy layer of the fibers can be collected using this setup.
Published date: 04 August 2020
Last updated: 30 January 2024
▼ Reference
-
Koozekonan A G, Esmaeilpour M R M, Kalantary S, Karimi A, Azam K, Golbabaei F. Fabrication and characterization of TiO2 and MWCNT coated electrospinning nanofibers for UV protection properties. MethodsX 2021; 8: 101354.
Open Access
-
Li D, Xu K, Niu Z, Zhang C. Annealing and Plasma Effects on the Structural and Photocatalytic Properties of TiO2 Fibers Produced by Electrospinning. Catalysts. 2022; 12(11):1441.
Open Access
-
Li J, Qiao H, Du Y, Chen C, Li X, Cui J, Kumar D, Wei Q. Electrospinning Synthesis and Photocatalytic Activity of Mesoporous TiO2 Nanofibers. The Scientific World Journal 2012; 2012: 154939. https://www.hindawi.com/journals/tswj/2012/154939/
-
Li Z, Zhang H, Zheng W, Wang W, Huang H, Wang C, MacDiarmid A G, Wei Y. Highly Sensitive and Stable Humidity Nanosensors Based on LiCl Doped TiO2 Electrospun Nanofibers. J. Am. Chem. Soc. 2008; 130: 5036.
-
Nealy S L, Severino C, Brayer W A, Stanishevsky A. Nanofibrous TiO2 produced using alternating field electrospinning of titanium alkoxide precursors: crystallization and phase development. RSC Adv., 2020; 10: 6840.
Open Access
-
Qi Q, Zhang T, Wang L. Improved and excellent humidity sensitivities based on KCl-doped TiO2 electrospun nanofibers. Applied Physics Letters 2008; 93: 023105.
-
Song C G, Koppala S K, Yoon J W. Characterization of electrospun TiO2 nanofibers and its enhanced photocatalytic property under solar light irradiation. Journal of Ceramic Processing Research 2013; 14: 653. http://jcpr.kbs-lab.co.kr/file/JCPR_vol.14_2013/JCPR14-6/01.pdf
-
Wang W, Yuan Q, Chi Y, Shao C L, Li N, Li X T. Preparation and Photocatalysis of Mesoporous TiO2 Nanofibers via an Electrospinning Technique. Chem. Res. Chinese Universities 2012; 28: 727.
-
Zhang H, Tian B, Xue J, Ding G, J Xi, Cao Y. Hierarchical non-woven fabric NiO/TiO2 film as an efficient anode material for lithium-ion batteries. RSC Adv., 2019; 9: 24682.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech