Introduction of antibacterial property to nanofibers have been tested for applications such as air filtration and wound healing. Several strategies to incorporate the antibacterial property has been used, from controlled release of the antibacterial drug, simple blending of antibacterial substance to chemical bonding to the nanofibers. The antibacterial property of the modified nanofiber is often tested against gram positive (eg. Staphylococcus aureus) and gram negative bacteria (eg. Escherichia coli, Klebsiella pneumoniae ) to determine its effectiveness.
Silver nanoparticles is well-known for its antibacterial property and electrospun nanofiber membrane containing silver nanoparticles have been shown to be effective in inhibiting bacteria growth [Yuan et al 2010]. Although incorporation of silver nanoparticles may be achieved through direct blending of into the electrospinning solution [Yuan et al 2010], an alternative method is to incorporate silver nitrate into the solution followed by photo-reduction using UV irradiation [Son et al 2006, Rujitanaroj et al 2010], thermal reduction at 80 °C [Xu et al 2006] or aging [Rujitanaroj et al 2008]. UV irradiation of silver nitrate incorporated nanofibers showed that the silver nanoparticles were distributed close to the surface of the nanofibers [Son et al 2006, Rujitanaroj et al 2010]. However, thermal reduction of silver nitrate showed uniform distribution of silver nanoparticles throughout the cross-section of the nanofibers [Xu et al 2006]. Lok et al [2007] showed that for silver nanoparticles to be effective in inhibiting bacterial growth, it must first be converted into its ionic form. A study of the release of Ag+ ions in nanoparticles uniformly distributed in nanofibers showed an initial burst release followed by sustained release [Xu et al 2006]. For UV reduced silver nanoparticles on the surface of the nanofibers, an initial burst release was also observed. Compared with unconverted Ag+ ions in the nanofiber, the initial release was greater in the UV reduced silver nanoparticles [Rujitanaroj et al 2010]. This could be due to migration of Ag+ form the core to the surface during the formation of the silver nanoparticles. Using poly(ether amide) as the carrier electrospun fiber material, Liang et al (2014) was able to incorporate only 0.15% AgNO3 into the material to achieve inhibition rate of >99.9% against E. coli and S. aureus after UV reduction of AgNO3 in Ag nanoparticles. With 0.05% AgNO3, the inhibition rate was 88% and 76% for E. coli and S. aureus respectively. Similarly, Nuge et al (2017) showed that Ag nanoparticles loaded electrospun gelatin fibers were less effective against gram positive S. aureus compared to gram negative Escherichia coli. The difference in efficacy has been attributed to the presence of a thick peptidoglycan layer below the cell membrane of gram positive bacteria that protects it from external stresses and possibly reduced the penetration of silver nanoparticle. Thermal decomposition study of the composite material showed that with increasing Ag content, the decomposition temperature was reduced. Therefore, lower amount of AgNO3 also helps to maintain the stability of the material. However, given the low concentration of Ag nanoparticles, the effectiveness of the anti-bacteria property of membrane over time needs to be determined. It is possible that leaching of Ag nanoparticles may render the membrane ineffective after the initial release. In terms of antibacterial and cytotoxicity, a study by Lin et al (2014) suggests that silver nanoparticles (AgNPs) formed by UV irradiation is preferred over heat treatment and AgNO3 with electrospun poly(vinyl alcohol) (PVA) as carrier. Reduction of AgNO3 in electrospun PVA to AgNPs using heat showed greater diameter dispersion. However, in UV irradiation, the AgNPs diameter was much smaller with narrower distribution. Smaller AgNPs are known to exhibit stronger antibacterial characteristic due to larger surface area for Ag ion release. All the PVA-AgNPs fibrous mat was shown to be effective in inhibiting S. aureus and E. coli. As expected, PVA-Ag@UV electrospun fibers showed the greatest biocidal effect against S. aureus, as no bacterial colonies was detect after only 30 minutes of incubation while small colonies were still found in the PVA-Ag@heat and PVA-Ag samples. Interestingly, the mat showed almost no cytotoxicity with PVA-AgNPs towards PA317 cell line. However, with PVA-AgNO3, there is a AgNO3 concentration dependent cytotoxicity. Jin et al (2018) investigated the potential use electrospun chitosan (CS) membrane incorporated with Ag-CaP as bone guided regeneration membrane. The release of Ag ions will help to prevent infection at the surgical site. Bone marrow stromal cells were used to test biocompatibility of the membrane. Cell viability were compared on electrospun chitosan (CS), CaP/CS and Ag-CaP/CS membrane. CaP/CS membrane showed the highest cell proliferation and Ag-CaP/CS membrane showed lower cell proliferation indicating some inhibition of the cells. However, electrospun pure CS membrane showed the lowest cell proliferation although CS is known to be biocompatible.
The release of antibacterial compounds may be adjusted by modifying the local environment. Zhao et al (2023) electrospun fibers made of polycaprolactone (PCL), silver nanoparticles (AgNPs) and black phosphorus (BP), with PCL being the carrier matrix, AgNPs with antibacterial functionality and photothermal BP. When the PCL/AgNPs/BP fibers were irradiated with 808 nm near infra-red (NIR) laser at 0.8 W cm-2, the temperature was able to increase to about 41 °C in less than 2 min. With the increase in the temperature, the release rate of drugs is expected to increase concurrently. In the absence of NIR, the release of AgNPs was about 62% at 14 days. With application of NIR irradiation and an expected temperature of 41 °C, the release rate increased to 89%. The released silver nanoparticles and silver ions were found to be effective in inhibiting E. colidrugged resistant MRSA bacteria. In particular with the application of NIR irradiation, the increased release of Ag+ disrupted bacterial biofilm and the local hyperthermia induced by the BP increases bacterial sensitivity which further inhibits the bacteria.
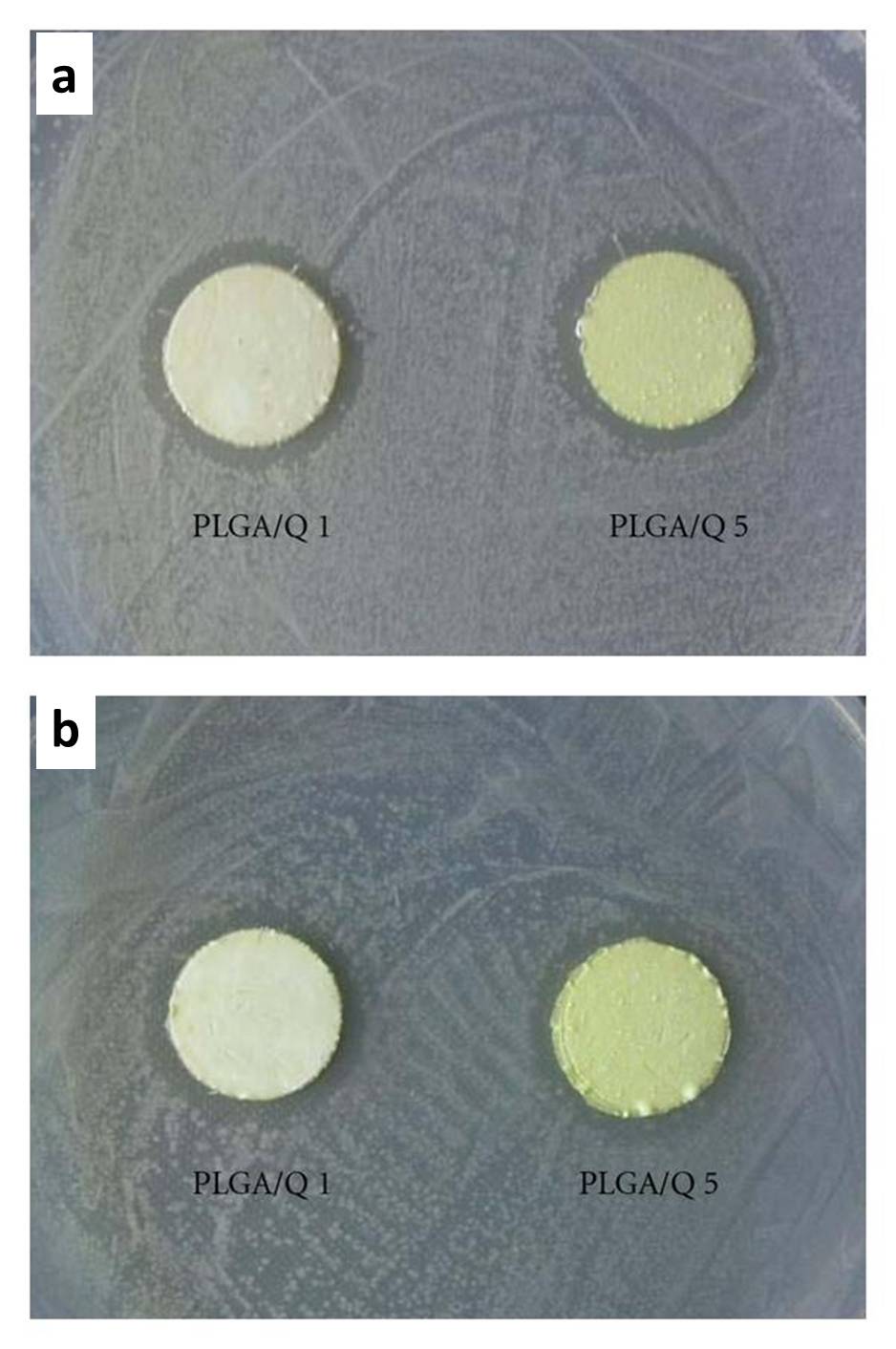
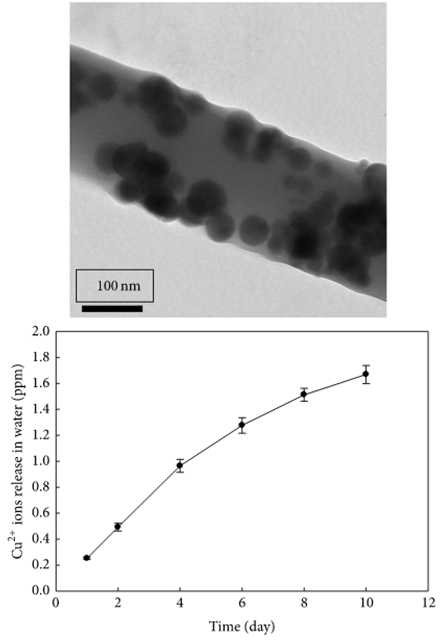
Apart from silver ions, other inorganic ions and nanoparticles which exhibit antibacterial properties have been incorporated into electrospun fibers. Haider et al (2015) showed that poly(lactide-co-glycolide) (PLGA) nanofibers containing 0.5wt% of CuO nanoparticles exhibited inhibition zones against E. coli and S. aureus while pure PLGA nanofibers does not show any inhibition zones. The antibacterial property of CuO was attributed to the release of Cu2+ ions. In water, the composite fibers showed gradual release of Cu2+ ions over ten days. NIH3T3 cells cultured on the PLGA and PLGA/CuO composite nanofiber scaffolds showed no significant difference in cytocompatibility.
At high concentration of Cu ions, cell viability may be affected. Unalan et al (2024) constructed cotton-wool-like 3D bioglass scaffolds doped with Zn and Cu from electrospinning of their precursors. Tests using HaCaT cells showed that Cu doped bioglass at ratio of 80Si-15Ca-5Cu having lower cell viability compared to bioglass with Zn at ratio of 80Si-15Ca-5Zn and 80Si-10Ca-5Zn-5Cu. Interestingly, the presence of Zn seemed to reduce the cytotoxicity of Cu in the bioglass. It has been hypothesized that the Zn ions may compete with the Cu ions for binding sites on the cell membrane which reduces the damaging effects of Cu.
Dhineshbabu et al (2014) used MgO nanoparticles loaded into nylon 6 solution for electrospinning fibers onto a cotton fabric. The resultant composite fabric showed significant improvement in its antibacterial property with 67% reduction against S. aureus and 63% reduction against E. coli. The presence of MgO nanoparticles in nanofibers also improves the flame retardancy performance of the cotton fabric as shown by the longer burning time at 18.5 s compared to just 6.3 s for uncoated cotton fabric. Nuge et al (2017) tested a wide range of metal nanoparticles, Cu-, Fe-, Ag-, Zn- and Ni- , loaded into electrospun gelatin fibers for antibacterial function against gram negative E. coli and gram positive S. aureus. Of these nanoparticles, Ni was found to be ineffective against bacteria as is the same for neat gelatin. Of the remaining nanoparticles, Fe and Cu nanoparticles were found to be more effective against gram positive S. aureus compared to gram negative E. coli. Greater efficacy of Cu towards gram positive bacteria may be attributed to greater abundance of amines and carboxyl groups on the cell surface of the bacterial which have greater affinity for copper. However, it is not immediately clear on the mechanism between Fe chelator inhibition of bacteria.
Feng (2019) tested the inhibition of gram-negative (E. coli) and gram-positive (S. aureus) bacteria under a fluorescent lamp source and UV-A (360 nm) irradiation by electrospun polylactic acid nanofibers loaded with TiO2 nanoparticles. At 0.75 wt% loading of TiO2 nanoparticles, zone of inhibitions were observed under both fluorescent lamp source and UV-A irradiation. However, the zone of inhibition under UV-A irradiation was significantly larger. TiO2 exhibited strong oxidising properties under long-wavelength ultraviolet light (320-400 nm) through the generation of reactive oxygen species (ROS) which potentially disrupts microbial functions leading to its death. TiO2 was found to be effective against both gram-positive and gram-negative bacteria but was more effective against gram-positive (S. aureus) bacteria.
The antimicrobial effect of compounds can be complex with some effective against only gram positive or gram negative bacteria. Combinations of antimicrobial compounds may also yield synergistic effects against some bacteria. Pleva et al (2025) loaded zein/polyethylene glycol (PEG) fibers with natural antimicrobial compounds and tested the antimicrobial effect against a selection of bacteria. Among single or combined loading of natural antimicrobial compounds, eugenol, thymol and nisin into the fibers, only the combination of eugenol and nisin were found to be effective against Gram-negative bacteria Escherichia coli, and Gram-positive bacteria Staphylococcus aureus and Listeria ivanovii. Against the formation of biofilms, only the combination of eugenol and thymol was able to prevent biofilm formation by all three bacteria. However, high concentration of eugenol and thymol (above 75%) extracted from the fibers was found to exhibit cell contact cytotoxic effects on NIH/3T3 cells. Other single or combinations of the antimicrobial compounds did not exhibit any cytotoxic effect.
Blending is current the most commonly used technique of incorporating antibacterial materials into the nanofiber. Fabricated membrane was shown to be effective in expressing antibacterial property. However, since the material is not chemically bonded to the polymer nanofiber, it is possible for the material to leach out of the membrane. N-halamine with different chemical structures have been tested for antibacterial effectiveness and leaching behaviour and it was found that chlorinated 3-dodecyl-5,5-dimethylhydantoin did not leach out despite immersion of the nanofiber membrane in its associate solvent over 24 h although chlorinated 5,5-dimethylhydantoin and chlorinated
2,2,5,5-tetramethyl-imidozalidin-4-one was detected over the same period [Tan et al 2007]. Since N-halamine has much lower solubility in water, leaching of the substance in water is not expected. Despite the stability of chlorinated 3-dodecyl-5,5-dimethylhydantoin to leaching, its antibacterial efficiency is less than chlorinated 5,5-dimethylhydantoin. A combination of antibacterial chemicals may also be added to the nanofibrous membrane to enhance its bacterial inhibition efficiency. A composite nanofibrous membrane consisting of Apatite, Ag, AgBr and TiO2 was shown to inactivate 99.9% of bacteria in 20 min under visible light [Wu et al 2009].
Antibacterial drugs have also been incorporated into electrospun nanofiber through blending. Electrospun polycaprolactone nanofiber yarn loaded with Ampicillin has been shown to be effective against Staphylococcus aureus and Klebsiella pneumonia. The drug release profile showed a high initial burst release with more than 75% of the drug depleted in the first hour and almost complete release in 96 h [Liu et al 2010B]. However, quercetin incorporated poly(l-lactide-co-glycolide) nanofiber was found to demonstrate a slow release rate with just 20% released after 16 days with a loading of 1 wt%. Growth inhibition of Staphylococcus aureus and Klebsiella pneumonia was shown at such low loading with good cell compatibility using epithelial cell [Xing et al 2012] . Other drugs such as gentamicin sulphate [Threepopnatkul et al 2010] and moxifloxacin [Shawki et al 2010] has also been added. In dental application, electrospun fibers may be used as a coating on dental implants to inhibit bacteria growth while potentially encouraging integration with host tissues. Baranowska-Korczyc et al (2016) loaded polycaprolactone (PCL) with ampicillin and tested for anti-bacterial activity against oral strain of Streptococcus sanguinis. The resultant electrospun membrane was found to inhibit bacteria growth through the release of ampicilin and minimal cytotoxicity from culturing of gingival fibroblast. There are many natural substance which exhibit antibacterial properties and these can be blended into polymer solution for electrospinning into fibers. Aruan et al (2017) tested the antibacterial performance of electrospun fibers comprising of a blend of ethanolic extract of soursop leaves (SLE) and polyvinyl alcohol (PVA). SLE concentration of 8%, 12%, and 14% loaded into PVA was found to be effective in inhibiting Staphylococcus aureus.
The progression of periodontitis was known to be caused by microorganisms, Aggregatibacter actinomycetemcomitans, Actinomyces viscosus, Prevotella intermedia, and Porphyromonas gingivalis. Therefore, any treatment for periodontitis and repairs should involve the inhibition of these microorganisms. Mirzaeei et al (2022) loaded tetracycline hydrochloride (TCH) into a polycaprolactone (PCL) solution for electrospinning into fibers. The resultant PCL/TCH fiber membrane showed antibacterial efficacy against all these species with 10 w/w% drug loading and greater inhibition zone with 20 w/w% loading. The highest efficacy was recorded against Actinomyces viscosus, and Porphyromonas gingivalis. With regards to the drug release profile, more than 60% of the drugs were released within the first 12 h and more than 90% was released after the next 144 h.
To introduce antibacterial property to a membrane, the use of antibiotics is the most direct method. However, frequent use of antibiotics have led to increasing antibiotic resistance. Other methods of preventing infections have been explored to reduce the reliance of antibiotics. Bao et al (2022) used a combination of graphene (Gr) and polyphenolic tannic acid (TA) in electrospun hyaluronic acid (HA) for the construction of a wound dressing. Gr is thought to exhibit antibacterial effects by physical puncturing and cutting, oxidative stress-induced damage, and photothermal effects. TA is also known to exhibit strong antibacterial properties and crosslink with Gr through hydrogen bonding or ions. Further TA can inhibit hyaluronidase activity, to enhance the stability of hyaluronic acid When tested against Staphylococcus aureus, HA/Gr membrane has an inhibition rate of 38% while pure HA membrane has an inhibition rate of only 8%. HA/Gr/TA membrane has the highest inhibition rate of 97%. Therefore, while Gr exhibits antibacterial property, the addition of TA is necessary to bring the antibacterial effect to over 90%. Biocompatibility tests were carried out using NIH-3T3 cells on a composition of HA/Gr/TA (20% w/v HA + 0.1% w/v Gr + 0.3% w/v TA) and compared with the control which were cells cultured in the well without any membrane. Their results showed that the proliferation of the cells on the HA/Gr/TA membrane was significantly better than the control over a 36 h period which provided evidence that the Gr at such low concentration may be safe.
Melt electrospinning offers many advantages over conventional solution-based electrospinning. The main advantage is that there is no need for the use of solvents which may pose an environmental hazard. To use melt electrospinning to produce fibers with antibacterial properties, the selection of the antibacterial additives is very important as melt electrospinning typically requires a high temperature to melt the polymer. Hence, the additives need to be tolerant of high temperature without losing its functionality after electrospinning. Li et al (2020) demonstrated the melt electrospinning of polypropylene (PP) with nano-ZnO particles as the antibacterial agent. Inorganic nanoparticles are suitable for blending into melt electrospinning polymers as they are able to tolerate higher temperature without loss of functionality. However, unlike solution electrospinning, a pelletizer or similar machine needs to melt the polymer and mix the inorganic additives into it prior to electrospinning. The resultant melt electrospun PP/ZnO fibers have an average diameter of 16 µm and was shown to inhibit Escherichia coli and Staphylococcus aureus. The inhibition mechanism of ZnO was thought to come from the release of Zn2+ which reacts with water to form oxidative radicals. These oxidative radicals would in turn attack the body of microorganisms thus killing them.
Lanasol is a naturally occurring brominated cyclic compound that can be extracted from red sea algae. Andersson et al (2014) used this compound to produce electrospun poly(methyl methacrylate) (PMMA) and polyethylene oxide (PEO) fibers with anti-bacterial properties. Various tests were performed to determine the efficacy of Lanasol loaded electrospun fibers in S. aureus. With more than 10 wt% Lanasol loading, dynamic contact measurement where the fibrous membrane was immerses in aqueous bacterial suspension under agitation, showed no viable count of bacteria colonies. Tests based on bacterial viability after adsorption onto fibrous membrane surface showed viability reduction of ca. 4 orders of magnitude (99.99% reduction) with just 4 wt % Lanasol loading.
An alternative method of incorporating nanoparticles to electrospun membrane using blending is concurrent electrospinning and spraying of nanoparticles. This has been used successfully to deposit TiO2 particles onto the surface of nylon-6 nanofibers. The adhesion of the particles was demonstrated to be strong enough to withstand sonication. However, it was found that nylon-6 can be degraded when exposed to more than 10 h of UV irradiation [Zhang et al 2013].
To release antibacterial agents in a more controlled manner, a core-shell fiber structure may be preferred over blending. Ye et al (2020) investigated the use of core-shell electrospun fibers for delivery of the drug, emodin. Emodin has been shown to be effective against Methicillin-resistant Staphylococcus aureus (MRSA). In its unencapsulated form, emodin is crystalline and hydrophobic which limits its usage. When emodin was blended into poly(vinylpyrrolidone) (PVP) solution and electrospun in a core-shell fiber structure, it existed in an amorphous form. The core of the fiber was made of hydrophilic PVP and emodin while the sheath was made of hygroscopic cellulose acetate (CA). In vitro drug release test showed that the dissolution rate of emodin from the nanofiber membranes was significantly higher than that of raw emodin. At 156 h, more than 99% of emodin has been released from the electrospun membrane but only 31% was dissolved from raw emodin. The emodin release profile showed a burst release of 49% in the first 0.5 h followed by sustained release over the next 6 days. The rapid release in the first 0.5 h may be due to higher concentration of emodin at the core-shell interface between the PVP and CA which migrates quickly through the CA matrix. Subsequent sustained release comes from emodin trapped in the PVP matrix. Tests on the efficacy of the emodin loaded membrane with MRSA showed clear inhibition for 24 h and up to 9 days without reduction in the inhibition zone size.
To improve the stability and retention of the additive in the nanofiber, attachment of the molecule to the polymer through covalent bonding may be used instead of physical blending. UV-grafting has been used to immobilize quaternary ammonium groups on the surface of the nanofibers [Yao et al 2008, Yao et al 2009]. However, such surface treatment has been shown to reduce the mechanical property of the nanofibrous membrane [Yao et al 2008].
In wound healing, a biocompatible polymer or additive with antibacterial property may be used. Chitosan is known to exhibit anti-bacterial properties and biocompatibility. While chitosan has been electrospun into nanofibers as a stand-alone material [Haider et al 2013], it is generally easier to produce fibers by blending with other polymers for electrospinning. Poly(ethylene terephthalate) with chitosan incorporated has been shown to inhibit the activity of Staphylococcus aureus and Klebsiella pneumonia while improving cell proliferation compared to poly(ethylene terephthalate) alone [Jung et al 2007]. Yuan et al (2016) showed that a minimum ratio of chitosan is needed for the composite to demonstrate anti-bacterial properties. In their experiment, a 2 to 1 ratio of chitosan to polyethylene oxide is needed for the composite to exhibit superior inhibition of both growth and attachment of Staphylococcus aureus. To demonstrate their biocompatibility, murine fibroblast was cultured on the scaffold and it showed good biocompatibility. Electrospun polycaparolactone nanofiber membrane with 1% wt silver-loaded zirconium phosphate nanoparticles (nanoAgZ) incorporated was also found to have no significant impact on the proliferation of dermal fibroblast while demonstrating excellent antibacterial performance with about 99% reduction in Staphylococcus aureus and Escherichia coli after 24 h [Duan et al 2007]. In vivo trial of polyvinyl alcohol coated with silver nanoparticles (by dipping membrane in 5 wt% AgNO3 followed by UV irradiation) using Sprague-Dawley rat full-thickness skin model does not exhibit any effect on wound healing compared to membrane without the antibacterial coating [Liu et al 2010A].
Antibacterial additives may be loaded in electrospun fibers in different ways to tailor the release of the additives. Zhou et al (2023) loaded an electrospinning device with a polyvinylpyrrolidone (PVP) solution containing zein/Yunnan Baiyao (YB) particles and ciprofloxacin (CIP). CIP is a broad spectrum antibacterial medication while YB is a traditional Chinese herbal preparation for treating bruises, injuries, and bleeding wounds. When applied on a wound, fast dissolution of PVP (within a minute) would release CIP for disinfection. Since zein/YB particles are insoluble in water, they were released when the PVP dissolved. Slower release of YB from zein over 12 h would facilitate hemostatic and myogenic effects for wound healing. YB also showed antibacterial effect through slow release from zein with longer incubation time leading to greater antibacterial effect. Inhibition of Wb800 and Escherichia coli increases from 88.3% to 99.9% and from 81.1% to 99.9% after 2 and 12 h, respectively. Therefore, having both CIP and YB encapsulated in different forms has the synergistic effect of addressing short term and longer term healing of the wound.
With electrospun membrane, the nanofiber structure may also enhance the antibacterial performance of the material. Samira et al (2020) compared the antibacterial performance between electrospun polyvinyl alcohol (PVA)/chitosan and film cast of the same material. Chitosan is the antibacterial agent while PVA was added to improve electrospinnability. Electrospun PVA/Chitosan membrane showed a higher antibacterial activity compared to PVA/Chitosan film cast based on colony forming unit (CFU) counts over 1 h, 8h and 24 h period. Electrospun PVA/Chitosan used in this study has an average diameter of 56.9 nm. Rod-shaped bacteria such as E. coli which was used in this study is in the micron size and its attachment to nanofiber structure in diameters smaller than its length may have led to conformational changes of the bacteria as it tries to wrap around each fiber. This may stress the bacteria and hence increases the antibacterial effect by nanofibers. In the study, the antibacterial effect on E. coli was stronger than S. aureus across all time points. Hydrophilicity of gram-negative bacteria made them more susceptible to damage by chitosan.
While blending may be one of the most common methods of introducing antibacterial properties to the electrospun fibers, there are other methods for introducing anti-bacterial properties to electrospun fibers. Sputtering is a common technique used for coating a surface with metal. There are several metals that exhibits natural natural.anti-bacterial properties and these can be sputtered on electrospun fiber membrane surface. Badaraev et al (2017) used DC magnetron sputtering of the copper target onto electrosoun poly-l-lactide acid polymer (PLLA) membranes. When tested against E. Coli, the copper coated membrane was able to cause a 50% reduction in the bacteria numbers. At 50% reduction, its performance pales in comparison with other antibacterial membranes. Instead of copper, silver may be sputtered on the electrospun fibers surface to incorporate antibacterial properties. Silver has already been sputtered on electrospun fibers for other applications such as improving its electrical conductivity [Memic et al 2017] thus it is just a matter of testing it for antibacterial performance.
Dip coating is another simple way of loading the surface of the electrospun fibers with antibacterial materials. Ye et al (2025) used a dip coating method to load electrospun poly(lactic-co-caprolactone)/gelatin (PLCL/GEL) fibers with Tannic acid (TA). TA is a natural polyphenol with strong antibacterial, antioxidant and anti-inflammatory properties.
Release of TA was rapid in the first 20 min followed by gradual and continuous release over the next two days. This release profile is favorable for initial inhibition of bacteria and subsequent maintenance of bacteria free enclosed environments. PLCL/GEL/TA demonstrated antibacterial property against E. coli and S. aureus. When tested as a packaging material for blueberries, the electrospun membrane without TA showed signs of decay within the first couple of days. With PLCL/GEL/TA(10 wt%), there was no color or structural deterioration after 5 days of storage except small patches of mold. Note that with 20wt% TA, the preservation performance was lower than with 10wt% TA and this may be attributed to greater hydrophilicity of the former which accumulated more moisture and condensation inside the package thereby accelerating food spoilage.
Researchers have also constructed electrospun fibers that produce antibacterial chemicals in the right condition. Leonarta and Lee (2021) used electrospun polyvinyl alcohol (PVA) nanofibrous to separately encapsulate glucose oxidase (GOx) and glucose (Glu). In aqueous media, GOx would catalyze the reaction between glucose released from the PVA/Glu nanofibers and oxygen to produce hydrogen peroxide (H2O2). H2O2 is a strong oxidising agent which is able to kill bacteria. The mix of PVA/Glu nanofibers and PVA/GOx nanofibers were able to give a sustained release of H2O2 over 7 days in room temperature. Cross-linking of the nanofiber membranes using glutaraldehyde (GA) vapor prevent the nanofibrous membrane from dissolving in water and prolonged the release of H2O2 probably due to slower release of glucose from the nanofibers. The sustained release of H2O2 was found to be effective against both Escherichia coli and Staphylococcus aureus with Gram(+) S. aureus cells being more susceptible to H2O2 than Gram(-) E. coli and & gt;99% of S. aureus were killed after 1 h incubation with the membrane. Such mixture of nanofibers containing an enzyme and the biomolecules have the potential to be used for wound healing in particular diabetic patients which has a higher level of blood glucose for the production of H2O2
Depending on the application, the antibacterial additive may even improve the electrospun fibers. Kim et al [2007] selected quaternary ammonium salt (benzyl triethylammonium chloride, BTEAC) to be blended with polycarbonate solution for electrospinning. The resultant fiber diameter was reduced significantly from more than 8 µm to about 1 µm with improved fiber uniformity. The electrospun membrane exhibited antibacterial property with good filtration performance.
Table 1. Electrospun nanofibers with antibacterial additives
| Antibacterial Additive
|
Polymer
|
Method of Incorporation
|
Bacteria tested (% reduction)
|
Reference
|
| Ampicillin
|
Polycaprolactone
|
Blending
|
Staphylococcus aureus, Klebsiella pneumoniae
|
Liu et al 2010B
|
| Benzyl triethylammonium chloride (quaternary ammonium salt)
|
Polycarbonate
|
Blending
|
Staphylococcus aureus (90.4% at 18 h), Klebsiella pneumonia (99.9% at 18 h), Escherichia coli (99.9% at 18 h)
|
Kim et al 2007B
|
| Chitosan
|
Poly(ethylene terephthalate)
|
Blending
|
Staphylococcus aureus (>95% at 24 h) and Klebsiella pneumonia (>90% at 24 h)
|
Jung et al 2007
|
| Gentamicin sulphate
|
Polylactic acid / polyethylene glycol
|
Blending
|
Staphylococcus aureus
|
Threepopnatkul et al 2010
|
| Moxifloxacin
|
Dextran
|
Blending
|
Staphylococcus aureus [99.9% at 12 h], Escherichia coli [100% at 12 h]
|
Shawki et al 2010
|
| N-halamine
|
Nylon-6
|
Blending
|
Staphylococcus aureus, Escherichia coli
|
Tan et al 2007
|
| Poly(4-vinyl-N-alkyl pyridinium bromide)
|
Poly(vinylidene fluoride-co-hexafluoropropylene)
|
UV graft copolymerization and quaternization
|
Staphylococcus aureus (99.9999% at 2 h), Escherichia coli (99.9999% at 2 h)
|
Yao et al 2009
|
| Poly(4-vinyl-N-hexyl pyridinium bromide)
|
Polyurethane
|
Plasma treatment followed by UV graft
|
Staphylococcus aureus (99.9% at 1 h, 99.999% at 4 h), Escherichia coli (99.9% at 4 h)
|
Yao et al 2008
|
| Quaternised chitosan
|
Polyvinyl alcohol
|
Blending
|
Staphylococcus aureus, Escherichia coli (98% at 2 h)
|
Ignatova et al 2006
|
| Quercetin dihydrate
|
Poly (l-lactide-co-glycolide)
|
Blending
|
Staphylococcus aures, Klebsiella pneumonia
|
Xing et al 2012
|
| Silver nitrate (AgNO3) and titanium dioxide (TiO2)
|
Chitosan/poly(vinyl alcohol)
|
Blending
|
Staphylococcus aureus (99%), Escherichia coli (98%)
|
Son et al 2009
|
| Silver nitrate reduced silver nanoparticles
|
Poly(vinyl alcohol)
|
Blending
|
Staphylococcus aureus (>99.9% at 18 h), Klebsiella pneumonia (>99.9% at 18 h)
|
Hong et al 2006
|
| Silver nitrate reduced silver nanoparticles
|
Cellulose Acetate
|
Blending
|
Staphylococcus aureus (99.9% at 18 h), Escherichia coli (99.9% at 18 h), Klebsiella pneumonia (99.9% at 18 h), Pseudomonas aeruginosa (99.9% at 18 h)
|
Son et al 2006
|
| Silver nitrate reduced silver nanoparticles
|
Poly(L-lactide)
|
Blending
|
Staphylococcus aureus (98.5% at 24 h), Escherichia coli (94.2% at 24 h)
|
Xu et al 2006
|
| Silver nitrate reduced silver nanoparticles
|
Polyvinyl alcohol
|
Coating (by dipping in AgNO3 solution)
|
Escherichia coli
|
Liu et al 2010A
|
| Silver nitrate reduced silver nanoparticles
|
Polyacrylonitrile
|
Blending
|
Staphylococcus aureus, Escherichia coli
|
Rujitanaroj et al 2010
|
| Silver nitrate reduced silver nanoparticles
|
Gelatin
|
Blending
|
Pseudomonas aeroginosa, Staphylococcus aureus, Escherichia coli, methicillin-resistant S. aureus
|
Rujitanaroj et al 2008
|
| Silver nanoparticles
|
Poly(vinylidene fluoride)
|
Blending
|
Staphylococcus aureus (77.7% at 24 h), Klebsiella pneumonia (77% at 24 h)
|
Yuan et al 2010
|
| Silver nanoparticles, AgBr, TiO2
|
-
|
Blending
|
Staphylococcus aureus, Escherichia coli (99.9% at 20 min)
|
Wu et al 2009
|
| Silver-loaded zirconium phosphate nanoparticles (nanoAgZ)
|
Polycaprolactone
|
Blending
|
Staphylococcus aureus, Escherichia coli
|
Duan et al 2007
|
| TiO2
|
Nylon-6
|
Simultaneous electrospinning and electrospraying
|
Escherichia coli
|
Zhang et al 2013
|
Beyond passive interception of bacteria and inactivating them, electrospun fibers may be made to facilitate their collection for inactivation. Riegier et al (2016) used electrospun cellulose membrane functionalized with polyelectrolytes, namely, polyacrylic acid (PA), chitosan (CS), and polydiallyldmethylammonium chloride (PDADMAC) and tested them for bacteria collection and inactivation. Using E. coli as the test subject, the study showed that hydrophilic membrane with neutral or positive surface charges showed the best bacteria collection. In this case unfunctionalized electrospun cellulose fiber and cellulose fiber coated with PDADMAC showed good bacteria collection and the latter demonstrated 97% bacteria inhibition after 180 minutes.
With increasing emphasis on reducing carbon footprint, there is a growing interest in using sustainable materials in electrospinning to produce nanofibrous products for various applications. Lee et al (2020) constructed an antibacterial membrane made of electrospun biopolyurethane. The biopolyurethane was made from castor oil and polycaprolactone-diol (PCL-diol). To construct the bio-based antibacterial membrane, triclosan (TR) was used as the antibacterial agent. To solubilise TR, α, β and γ-cyclodextrin (CD) was tested as a TR carrier in the biopolyurethane matrix. Their test showed that only γ-cyclodextrin (CD) forms a full complex with TR for maximum solubility. Tests against S. aureus and K. pneumonia showed that biopolyurethane/TR/γ-CD cytostatic efficiency of more than 99.9%. Without γ-CD, the cytostatic efficiency was 97.0% and 98.1% against S. aureus and K. pneumonia respectively. Hence, the presence of γ-CD helps in the solubilising and release of TR in the media.
The antibacterial property of an electrospun membrane may also be affected by the loading efficiency. Zhao et al (2019) used polydopamine (PDA) as bonding agent on electrospun poly(lactic-co-glycolic acid) (PLGA) fibers for surface adhesion of fibroblast growth factor (bFGF) and antibacterial agent, ponericin G1. Ponericin G1 is a natural antibacterial peptide extracted from ants. PDA is a molecule found in the adhesive proteins of mussel and can improve hydrophilicity and functionalize the surface coated with it. The antibacterial effect of ponericin G1 loaded on PLGA/PDA fibers was also found to be greater than ponericin G1 loaded on PLGA fibers when tested against S. aureus and E. coli. 16 hours of culture on nutrient agar showed significant inhibition against S. aureus and E. coli with a greater zone of inhibition on PLGA/PDA/ponericin G1 compared to PLGA/ponericin G1 and PLGA only scaffolds. This shows the greater loading efficiency of ponericin G1 in the presence of PDA.
A less commonly investigated property of antibacterial membrane is the effect of pH and temperature on its ability to inhibit bacterial growth. This is especially important for material that is sensitive to either temperature or pH. Wei et al (2021) used poly(N-isopropyl acrylamide- N-Methylol acrylamide-acrylic acid) (PNIPAm-NMA-Ac) as the material for drug loading and electrospinning into fibrous membranes. The drug release rate from electrospun PNIPAm-NMA-Ac fibrous membrane is dependent on both thermal and pH of the environment. This is because this copolymer is made of poly(N-isopropyl acrylamide) (PNIPAm) which is thermo-responsive, switching between hydrophilic and hydrophobic forms at 32 °C. The other part of the copolymer is poly(acrylic acid) (PAc) which is pH-sensitive and extends and shrinks at pH above and below 4.75 respectively. Two antimicrobial drugs, gatifloxacin hydrochloride (GH) and silver nanoparticles (Ag) were incorporated in the electrospun fibrous membrane. GH is by blending and Ag is by post-spinning reduction of Ag salt which was loaded into the solution prior to electrospinning. At a temperature of 20°C which is below the lower critical solution temperature (LCST) of PNIPAm-NMA-Ac, swelling of the fibers reduced the release of GH and Ag. Maximum release of GH and Ag is at temperature above 37°C or pH 4.0. Due to the influence of drug release from the polymer matrix material by temperature and pH, it was found that PNIPAm-NMA-Ac GH ?+ ?Ag fibrous membranes exhibited antibacterial activity against E. coli of 97. 7% and 95.2% at 37 °°C and pH 6.8 or pH 4.0. At a low temperature of 20 °C and a more alkaline condition of pH 10, the reduced release of both GH and Ag is reflected by a reduction of antibacterial activity to 59.4% and 3.5% respectively. The antibacterial influence of temperature and pH was similar when tested against S. aureus.
Published date: 29 November 2013
Last updated: 23 December 2025
▼ Reference
-
Andersson R L, Martínez-Abad A, Lagaron J M, Gedde U W, Mallon P E, Olsson R T,Hedenqvist M S. Antibacterial Properties of Tough and Strong Electrospun PMMA/PEO Fiber Mats Filled with Lanasol-A Naturally Occurring Brominated Substance. Int J Mol Sci. 2014; 15(9): 15912 - 15923.
Open Access
-
Aruan N M, Sriyanti I, Edikresnha D, Suciati T, Munir M M, Khairurrijal. Polyvinyl Alcohol/Soursop Leaves Extract Composite Nanofibers Synthesized Using Electrospinning Technique and their Potential as Antibacterial Wound Dressing. Procedia Engineering 2017;170: 31.
Open Access
-
Badaraev A D, Nemoykina A L, Bolbasov E N,Tverdokhlebov S I. PLLA scaffold modification using magnetron sputtering of the copper target to provide antibacterial properties. Resource-Efficient Technologies 2017; 3(2):204
Open Access
-
Baranowska-Korczyc A, Warowicka A A, Jasiurkowska-Delaporte M, Grzeskowiak B, Jarek M, Maciejewska B M, Jurga-Stopa J, Jurga S. Antimicrobial electrospun poly(ε-caprolactone) scaffolds for gingival fibroblast growth. RSC Adv. 2016; 6: 19647.
-
Bao X, Zhu Q, Chen Y, Tang H, Deng W, Guo H, Zeng L. Antibacterial and antioxidant films based on HA/Gr/TA fabricated using electrospinning for wound healing. International Journal of Pharmaceutics 2022; 626: 122139.
Open Access
-
Dhineshbabu N Rm Karunakaran G, Suriyaprabha R, Manivasakan P, Rajendran V. Electrospun MgO/Nylon 6 Hybrid Nanofibers for Protective Clothing. Nano-Micro Letters 2014; 6: 46.
Open Access
-
Duan Y Y, Jia J, Wang S H, Yan W, Jin L, Wang Z Y. Preparation of Antimicrobial Poly(e-caprolactone) Electrospun Nanofibers Containing Silver-Loaded Zirconium Phosphate Nanoparticles. J Appl Poylm Sci 2007; 106: 1208.
-
Feng S, Zhang F, Ahmed S, Liu Y. Physico-Mechanical and Antibacterial Properties of PLA/TiO2 Composite Materials Synthesized via Electrospinning and Solution Casting Processes. Coatings 2019; 9(8): 525.
Open Access
-
Haider S, Al-Zeghayer Y, Ali F A A, Haider A, Mahmood A, Al-Masry W A, Imran M, Aijaz M O. Highly aligned narrow diameter chitosan electrospun nanofibers. J Polym Res 2013; 20: 105.
-
Haider A, Kwak S, Gupta K C, Kang I K. Antibacterial Activity and Cytocompatibility of PLGA/CuO Hybrid Nanofiber Scaffolds Prepared by Electrospinning. Journal of Nanomaterials 2015; 2015: 832762.
Open Access
-
Hong K H, Park J L, Sul I H, Youk J H, Kang T J. Preparation of Antimicrobial Poly(vinyl alcohol) Nanofibers Containing Silver Nanoparticles. Journal of Polymer Science: Part B: Polymer Physics 2006; 44: 2468.
-
Ignatova M, Starbova K, Markova N, Manolova N, Rashkov I. Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly(vinyl alcohol). Carbohydr Res 2006; 341: 2098.
-
Jin S, LI J, Wang J, Jiang J, Zhou Y, Li Y, Yang F. Electrospun silver ion-loaded calcium phosphate/chitosan antibacterial composite fibrous membranes for guided bone regeneration. International Journal of Nanomedicine 2018; 13: 4591.
Open Access
-
Jung K H, Huh M W, Meng W, Yuan J, Hyun S H, Bae J S, Hudson S M, Kang I K. Preparation and Antibacterial Activity of PET/Chitosan Nanofibrous Mats Using an Electrospinning Technique. J Appl Polym Sci 2007; 105: 2816.
-
Kim S J, Nam Y S, Rhee D M, Park H S, Park W H. Preparation and characterization of antimicrobial polycarbonate nanofibrous membrane. European Polymer Journal 2007; 43: 3146.
-
Lee J H, Park S H, Kim S H. Fabrication of bio-based polyurethane nanofibers incorporated with a triclosan/cyclodextrin complex for antibacterial applications. RSC Adv. 2020; 10: 3450.
Open Access
-
Leonarta F and Lee C K. Nanofibrous Membrane with Encapsulated Glucose Oxidase for Self-Sustained Antimicrobial Applications. Membranes (Basel). 2021; 11(12): 997.
Open Access
-
Li Q S, He H W, Fan Z Z, Zhao R H, Chen F X, Zhou R, Ning X. Preparation and Performance of Ultra-Fine Polypropylene Antibacterial Fibers via Melt Electrospinning. Polymers (Basel). 2020; 12(3): 606.
Open Access
-
Liang S, Zhang G, Min J, Ding J, Jiang X. Synthesis and Antibacterial Testing of Silver/Poly (Ether Amide) Composite Nanofibers with Ultralow Silver Content. Journal of Nanomaterials 2014; 2014: 684251.
Open Access
-
Lin S, Wang R, Yi Y, Wang Z, Hao L, Wu J, Hu G, He H. Facile and green fabrication of electrospun poly(vinyl alcohol) nanofibrous mats doped with narrowly dispersed silver nanoparticles. International Journal of Nanomedicine 2014; 9: 3937.
Open Access
-
Liu X, Lin T, Fang J, Yao G, Zhao H, Dodson M, Wang X. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. J Biomed Mater Res Part A 2010; 94A: 499. [A]
-
Liu H, Leonas K K, Zhao Y. Antimicrobial Properties and Release Profile of Ampicillin from Electrospun Poly(?-caprolactone) Nanofiber Yarns. Journal of Engineered Fibers and Fabrics 2010; 5: 10 [B]
Open Access
-
Lok C N, Ho C M, Chen R, He Q Y, Yu W Y, Sun H, Tam P W H, Chiu J F, Che C M. Silver nanoparticles: partial oxidation and antibacterial activities. Journal of Biological Inorganic Chemistry 2007; 12: 527.
-
Memic A, Aldhari M, Tamayol A, Mostafalu P, Abdel-wahab M S, Samandari M, Moghaddam K M, Annabi N, Bencherif S A, Khademhosseini A. Nanofibrous Silver-Coated Polymeric Scaffolds with Tunable Electrical Properties. Nanomaterials 2017; 7: 63.
Open Access
-
Mirzaeei S, Moghadam F, Asare-Addo K, Nokhodchi A. Design of a nanofibrous guided tissue regeneration carrier as a potential drug delivery system for tetracycline hydrochloride in the management of periodontitis. Journal of Drug Delivery Science and Technology 2022; 75: 103722.
Open Access
-
Nuge T, Tshai K Y, Lim S S, Nordin N, Hoque M E. Preparation and characterization of Cu-, Fe-, Ag-, Zn- and Ni- doped gelatin nanofibers for possible applications in antibacterial nanomedicine. Journal of Engineering Science and Technology 5th EURECA 2015 Special Issue March 2017; 3: 68.
Open Access
-
Pleva P, Bartosová L, Janalíková M, Polásková M, Sisková A O, Matosková L, Krejcí O, Sedlaríková J. Biodegradable zein/PEG nanofibers incorporated with natural antimicrobial compounds for eco-friendly food packaging. New Biotechnology 2025; 88: 12.
https://www.sciencedirect.com/science/article/pii/S1871678425000366 Open Access
-
Rieger K A, Porter M, Schiffman J D. Polyelectrolyte-Functionalized Nanofiber Mats Control the Collection and Inactivation of Escherichia coli. Materials 2016; 9: 297.
-
Rujitanaroj P O, Pimpha N, Supaphol P. Preparation, Characterization, and Antibacterial Properties of Electrospun Polyacrylonitrile Fibrous Membranes Containing Silver Nanoparticles. J Appl Polym Sci 2010; 116: 1967.
-
Rujitanaroj P O, Pimpha N, Supaphol P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008; 49: 4723.
-
Samira N, Amir M M, Mohammad K G, Alireza S A. Preparation of PVA/Chitosan samples by electrospinning and film casting methods and evaluating the effect of surface morphology on their antibacterial behavior. 2020 Mater. Res. Express; 7: 015401.
Open Access
-
Shawki M M, Hereba A M, Ghazal A. Formation and Characterization of antimicrobial dextran nanofibers. Romanian J Biophys. 2010; 20: 335.
Open Access
-
Son W K, Youk J H, Park W H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydrate Polymers 2006; 65: 430.
-
Son B, Yeom B Y, Song S H, Lee C S, Hwang T S. Antibacterial Electrospun Chitosan/Poly(vinyl alcohol) Nanofibers Containing Silver Nitrate and Titanium Dioxide. J. Appl. Polym. Sci. 2009; 111: 2892.
-
Tan K, Obendorf S K. Fabrication and evaluation of electrospun nanofibrous antimicrobial nylon 6 membranes. Journal of Membrane Science 2007; 305: 287.
-
Threepopnatkul P, Vichitochote K, Saewong S, Tangsupa-anan T, Kulsetthanchalee C, Suttiruengwong S. Mechanical and Antibacterial Properties of Electrospun PLA/PEG Mats. Journal of Metals, Materials and Minerals 2010; 20: 185.
-
Unalan I, Rimoli I H, Mutlu N, Michálek M, Abraham G A, Liverani L, Boccaccini A R. Cotton wool-like ion-doped bioactive glass nanofibers: investigation of Zn and Cu combined effect. Biomed. Mater. 2024; 19: 065001.
https://iopscience.iop.org/article/10.1088/1748-605X/ad7084 Open Access
-
Wei Z, Yang J, Long S, Zhang G, Wang X. Smart and in-situ formation electrospun fibrous membrane for the control of antimicrobial efficacy. Smart Materials in Medicine 2021; 2: 87.
Open Access
-
Wu Y, Jia W, An Q, Liu Y, Chen J, Li G. Multiaction antibacterial nanofibrous membranes fabricated by electrospinning: an excellent system for antibacterial applications. Nanotechnology 2009; 20: 245101.
-
Xing Z C, Meng W, Yuan J, Moon S, Jeong Y, Kang I K. In Vitro Assessment of Antibacterial Activity and Cytocompatibility of Quercetin-Containing PLGA Nanofibrous Scaffolds for Tissue Engineering. Journal of Nanomaterials 2012; 2012: 202608.
Open Access
-
Xu X, Yang Q, Wang Y, Yu H, Chen X, Jing X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. European Polymer Journal 2006; 42: 2081.
-
Yao C, Li X, Neoh K G, Shi Z, Kang E T. Antibacterial activities of surface modified electrospun poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) fibrous membranes. Applied Surface Science 2009; 255: 3854.
-
Ye P, Wei S, Luo C, Wang Q, Li A, Wei F. Long-Term Effect against Methicillin-Resistant Staphylococcus aureus of Emodin Released from Coaxial Electrospinning Nanofiber Membranes with a Biphasic Profile. Biomolecules. 2020; 10(3): 362.
Open Access
-
Ye Y, Liu J, Yan T, Yan Y, Fan J, Zhang X, Gan L, Chai H, Zhou G. Tannic acid cross-linked Poly(lactic-co-caprolactone/Gelatin electrospun nanofibrous membranes for blueberry preservation. LWT 2025; 217: 117388.
https://www.sciencedirect.com/science/article/pii/S0023643825000726 Open Access.
-
Yuan J, Geng J, Xing Z, Shen J, Kang I K, Byun H. Electrospinning of Antibacterial Poly(vinylidene fluoride) Nanofibers Containing Silver Nanoparticles. J Appl Polym Sci 2010; 116: 668.
-
Yuan T T, Jenkins P M, Foushee A M D, Jockheck-Clark A R, Stahl J M. Electrospun Chitosan/Polyethylene Oxide Nanofibrous Scaffolds with Potential Antibacterial Wound Dressing Applications. Journal of Nanomaterials 2016; 2016: 6231040.
Open Access
-
Zhang Y, Lee M W, An S, Sinha-Ray S, Khansari S, Joshi B, Hong S, Hong J H, Kim J J, Pourdeyhimi B, Yoon S S, Yarin A L. Antibacterial activity of photocatalytic electrospun titania nano?ber mats and solution-blown soy protein nano?ber mats decorated with silver nanoparticles. Catalysis Communications 2013; 34: 35.
-
Zhao J, Han F, Zhang W, Yang Y, You D, Li L. Toward improved wound dressings: effects of polydopamine-decorated poly(lactic-co-glycolic acid) electrospinning incorporating basic fibroblast growth factor and ponericin G1. RSC Adv. 2019; 9: 33038.
Open Access
-
Zhao Y, Liu Y, Tian C, Liu ZZ, Wu K, Zhang C, 7an X. Construction of antibacterial photothermal PCL/AgNPs/BP nanofibers for infected wound healing. Materials & Design 2023; 226: 111670.
Open Access
-
Zhou J, Wang L, Gong W, Wang B, Yu D-G, Zhu Y. Integrating Chinese Herbs and Western Medicine for New Wound Dressings through Handheld Electrospinning. Biomedicines. 2023; 11(8):2146.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech