▼ Reference
- Chen S, John J V, McCarthy A, Carlson M A, Li X, Xie J. Fast transformation of 2D nanofiber membranes into pre-molded 3D scaffolds with biomimetic and oriented porous structure for biomedical applications. Appl. Phys. Rev. 2020; 7: 021406.
- Chronakis I S, Grapenson S, Jakob A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymer 2006; 47: 1597.
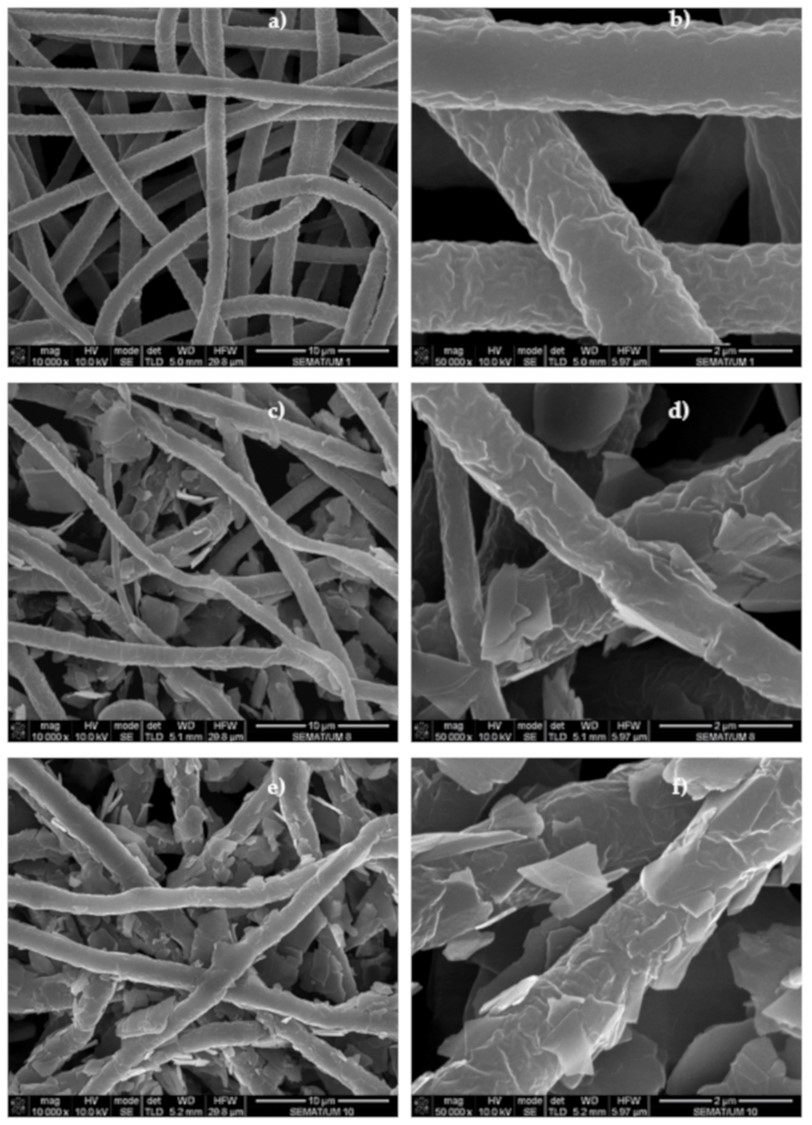
- Codau T C, Codau E. Synthesis of ultra-stretchable thermoelectric nanofibrous membrane based on wet-electrospun Polyurethane/MWCNTs composites. Materials Today Sustainability 2024; 27: 100831. https://www.sciencedirect.com/science/article/pii/S2589234724001672Open Access
- Dong H, Prasad S, Nyame V, Jones Jr. W E. Sub-micrometer Conducting Polyaniline Tubes Prepared from Polymer Fiber Templates. Chem. Mater. 2004; 16: 371.
- Francavilla P, Ferreira DP, Araújo JC, Fangueiro R. Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets. Applied Sciences. 2021; 11(3):1124. Open Access
- Granato F, Bianco A, Bertarelli C, Zerbi G. Composite Polyamide 6/Polypyrrole Conductive Nanofibers. Macromol. Rapid Commun. 2009; 30: 453.
- Han G, Guo B, Zhang L, Yang B. Conductive Gold Films Assembled on Electrospun Poly(methyl methacrylate) Fibrous Mats. Adv. Mater. 2006; 18: 1709.
- Hong K H, Oh K W, Kang T J. Preparation of Conducting Nylon-6 Electrospun Fiber Webs by the In Situ Polymerization of Polyaniline. J Appl Polym Sci 2005; 96: 983.
- Hou X, Yang X, Zhang L, Waclawik E, Wu S. Stretching-induced crystallinity and orientation to improve the mechanical properties of electrospun PAN nanocomposites. Materials and Design 2010; 31: 1726.
- Hyun T S, Kim H G, Kim I D. Facile synthesis and electrochemical properties of conducting SrRuO3RuO2 composite nanofibre mats. Journal of Power Sources 2010; 195: 1522.
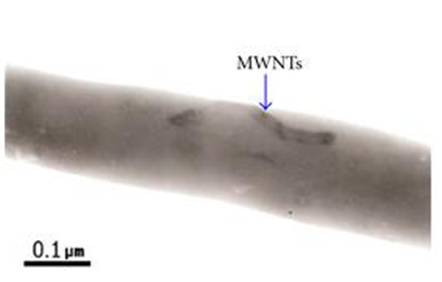
- Jeong J S, Jeon S Y, Lee T Y, Park J H, Shin J H, Alegaonkar P S, Berdinsky A S, Yoo J B. Fabrication of MWNTs/nylon conductive composite nanofibers by electrospinning. Diamond and related Materials 2006; 15: 1839.
- Jun I, Jeong S, Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials 2009; 30: 2038.
- Kang M, Jin H J. Electrically conducting electrospun silk membranes fabricated by adsorption of carbon nanotubes. Colloid Polym Sci 2007; 285: 1163.
- Laforgue A, Robitaille L, Mokrini A, Ajji A. Fabrication and Characterization of Ionic Conducting Nanofibers. Macromol. Mater. Eng. 2007; 292: 1229.
- Nair S, Natarajan S, Kim S H. Fabrication of Electrically Conducting Polypyrrole-Poly(ethylene oxide) Composite Nanofibers. Macromol. Rapid Commun. 2005; 26: 1599.
- Nair S, Hsiao E, Kim S H. Melt-Welding and Improved Electrical Conductivity of Nonwoven Porous Nanofiber Mats of Poly(3,4-ethylenedioxythiophene) Grown on Electrospun Polystyrene Fiber Template. Chem. Mater. 2009; 21: 115. Pol V G, Koren E, Zaban A. Fabrication of Continuous Conducting Gold Wires by Electrospinning. Chem. Mater. 2008; 20: 3055.
- Ra E J, Hyeok K, Kim K K, Jeong S Y, Lee Y H. Anisotropic electrical conductivity of MWCNT/PAN nanofiber paper. Chemical Physics Letters 2005; 413: 188.
- Shin M K, Kim Y J, Kim S I, Kim S K, Lee H, Spinks G M, Kim S J. Enhanced conductivity of aligned PANi/PEO/MWNT nanofibers by electrospinning. Sensors and Actuators B 2008; 134: 122.
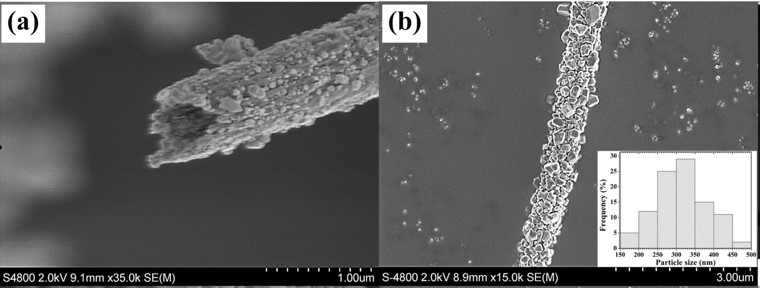
- Sirimekanont T, Supaphol P, Sombatmankhong K. Titanium (IV) oxide composite hollow nanofibres with silver oxide outgrowth by combined sol-gel and electrospinning techniques and their potential applications in energy and environment. Adv Compos Hybrid Mater 2023; 6: 115. Open Access
- Sundaray B, Subramanian V, Natarajan T S, Krishnamurthy K. Electrical conductivity of a single electrospun fiber of poly(ethyl methacrylate) and multiwalled carbon nanotube nanocomposite. Applied Physcs Letters 2006; 88: 143114.
- Weerasinghe V T, Dissanayake D G K, W. Pereira P T D, Tissera N D, Wijesena R N, Wanasekara N D. All-organic, conductive and biodegradable yarns from core-shell nanofibers through electrospinning. RSC Adv. 2020; 10: 32875. Open Access
- Wei M, Lee J, Kang B, Mead J. Preparation of Core-Sheath Nanofibers from Conducting Polymer Blends. Macromol. Rapid Commun. 2005; 26: 1127.
- Xuyen N T, Ra E J, Geng H Z, Kim K K, An K H, Lee H L. Enhancement of Conductivity by Diameter Control of Polyimide-Based Electrospun Carbon Nanofibers. J Phys Chem B 2007; 111: 11350.
▼ Credit and Acknowledgement
Author
Wee-Eong TEO View profile
Email: weeeong@yahoo.com
 ElectrospinTech
ElectrospinTech