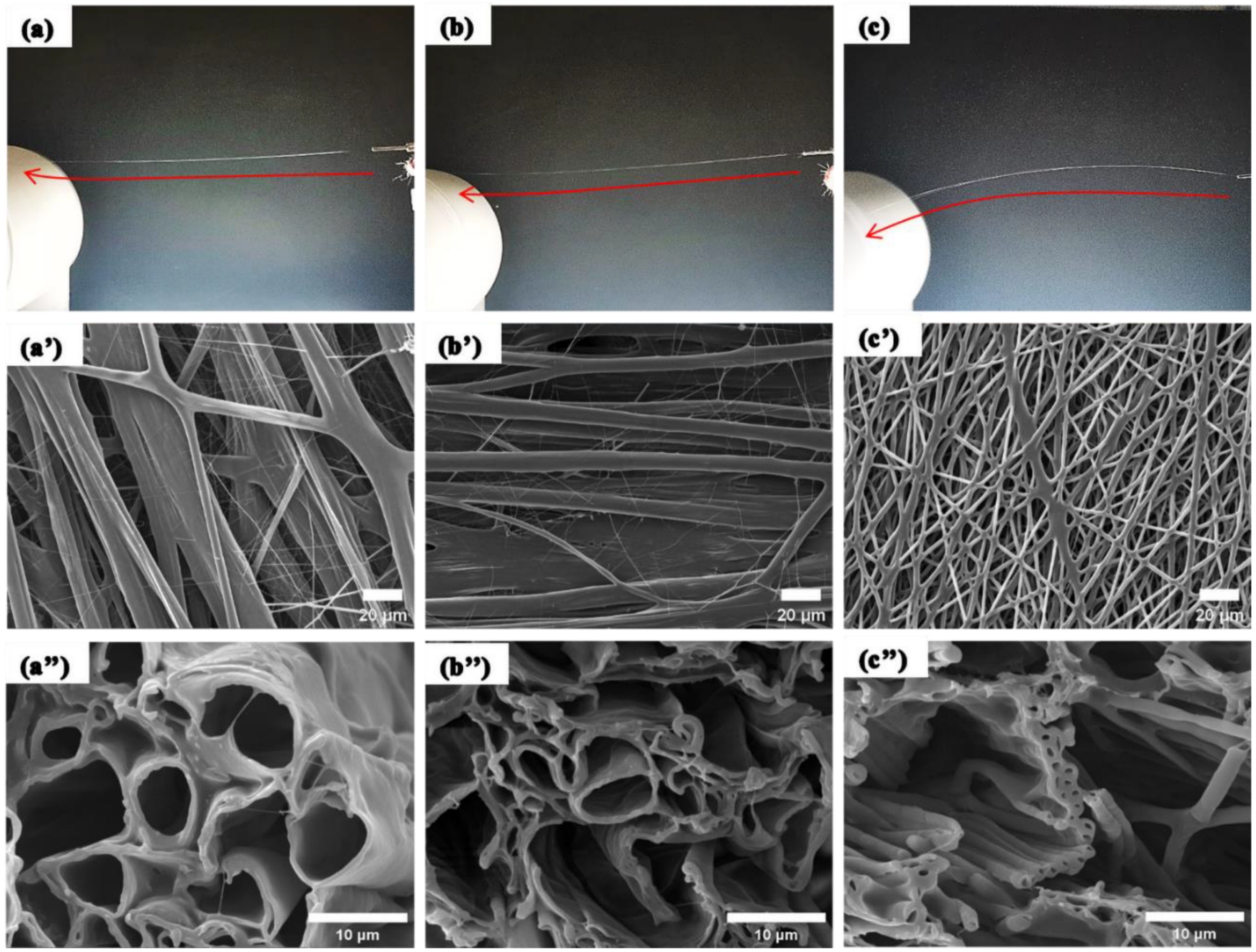
Electrospinning has been shown to be very versatile in constructing nanofibers from a wide range of materials. Nevertheless, there are some materials that cannot be electrospun due to low molecular weight, lack of suitable solvent or other reasons. Core-shell electrospinning is able to use an electrospinnable material as a carrier to produce nanofibers for non-electrospinnable material. This is very useful in application where the targeted material is of low molecular weight such as drugs. Another use of core-shell electrospinning is to construct hollow tube fibers. Where two miscible solutions are used in core-shell electrospinning, there is a possibility that there will be mixing of the two solutions are the boundaries. However, considering the time for diffusion and the bending instability time characteristics, it is estimated that the diffusion time taken is much longer than the bending instability stretching thus the sharp boundaries shall retain in core-shell electrospinning [Sun et al 2003].
To verify the effect of solution miscibility in the outcome of electrospinning core-shell fibers, Li et al (2024) used a polycaprolactone (PCL) solution as the shell solution and polyethylene oxide (PEO) solution with different solvents as the core. Using solvents that are polar and non-polar to dissolve PEO, they were able to construct PEO solution with differing miscibility with PCL solution. With immiscible and partially miscible core and shell solution, clear hollow fibers were observed after removal of the core PEO. However the fiber diameters were large with the fiber diameters from immiscible and partially miscible core and shell solution at 9 µm and 7 µm respectively. With completely miscible core and shell solution, hollow fibers were still obtained after removal of the core PEO but the diameter of the fibers were much smaller at 2.4 µm. The difference in diameter may be due to partial solidification at the interface between immiscible core and shell solutions which would resist stretching and resulting in larger diameters. This would not happen for miscible core and shell solutions which allowed the jet to stretch and solidify due to vaporization of the solvents.
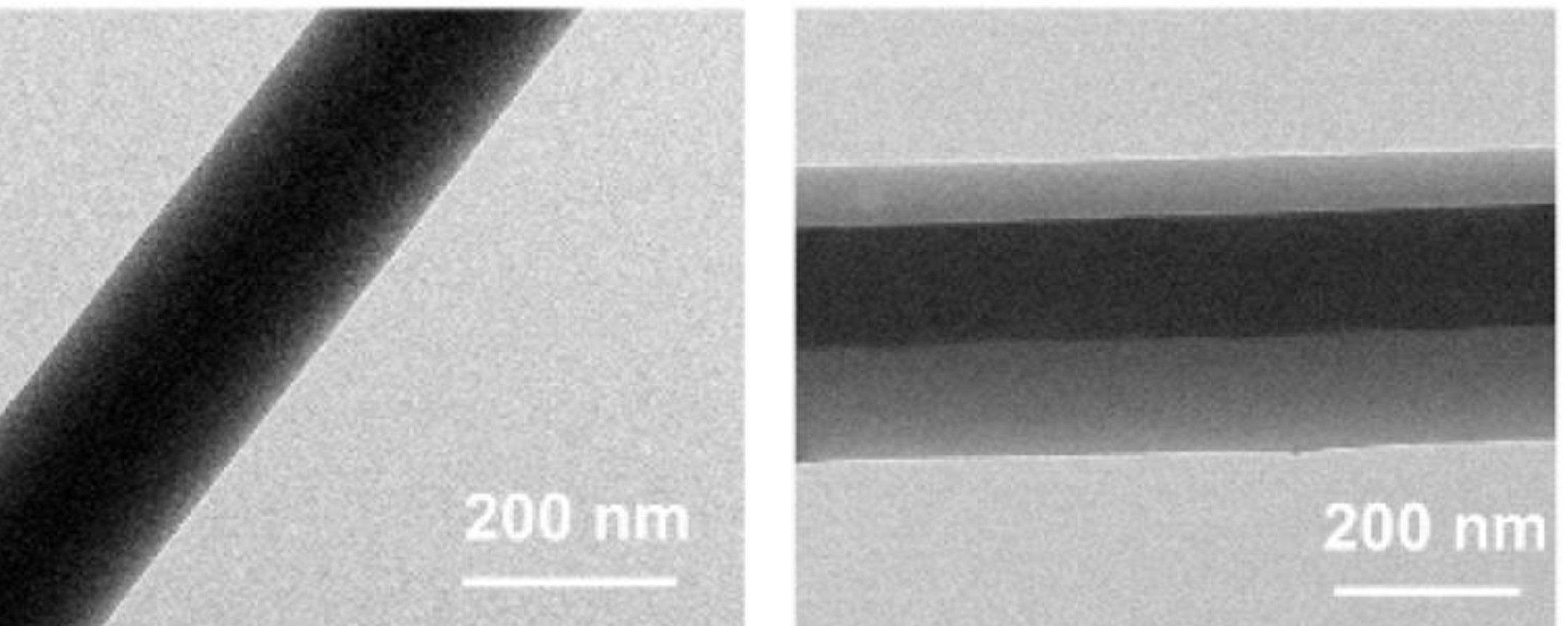
Coaxial electrospinning has also been used successfully to construct single polymer composite where the core and shell material are chemically similar. Kriel et al (2012) fabricated such composite with poly(L-lactide) as the core material and poly(DL-lactide) as the shell. Conventional method of viewing the core-shell profile using transmission electron microscope (TEM) is not possible due to poor or no contrast between the two materials. Indirect method involving removal of one of the component may be necessary to verify the core-shell arrangement.
In drug release applications, researchers have successfully electrospun core-shell fibers with the same material but with drugs loaded only in the core.
Reise et al (2023) used coaxial electrospun poly(L-lactide-co-D,L-lactide) (PLA) fibers mats for sustained release of metronidazole (MNA) in periodontitis treatment. Both the core and sheath material were PLA but MNA was only added to the core PLA solution. The resultant coaxial fibers (COAX) mat was compared with homogeneously distributed MNA in PLA nanofibers (HDN). The HDN showed an initial burst release but this was significantly reduced in the COAX which showed a more sustained release. Against F. nucleatum, both HDN and COAX fibers showed good inhibition from aliquots up to 168 h with COAX fiber showing greater inhibition than HDN after the first couple of hours. For P. gingivalis, antibacterial effect was shown up to 672?h for both HDN and COAX fibers however, COAX fibers showed significantly higher antibacterial effect at the time point. Cytocompatibility tests using human gingival fibroblasts (HGF) showed both mats to be compatible with COAX mat demonstrating significant higher numbers of viable cells. Therefore with a more sustained drug release from coaxial fibers, its antibacterial efficacy can be maintained with less stress on the cells.
Liu et al (2022) electrospun core-shell cellulose acetate (CA) with CA loaded with curcumin at the core. Instead of using a coaxial nozzle, a triaxial nozzle was used and the outermost layer was a solvent mixture of ethanol, DMF, and acetone. The outermost coverage by the solvents help to maintain the stability of the electrospinning process. The flow rate of the middle section containing pure CA was varied to control the thickness of the wall covering the innermost CA loaded fibers. TEM showed that the drug is localized at the core of the fiber.
Traditionally, a co-axial nozzle is used to spin core-shell fibers, however, it was later found that emulsion electrospinning was also able to spin core-shell fibers.
Carrier for non-electrospinnable material
Core-shell electrospinning only requires one of the components to be electrospinnable to form fibers. In coaxial nozzle electrospinning, the electrospinnable material is commonly used as the shell material to induce a one-dimensional arrangement to the core material. Conductive polymers such as polyaniline and polypyrrole which are known for their difficulty to form fibers has been electrospun to form fibers using coaxial electrospinning [Zhang and Rutledge 2012, Srivastava et al 2008].
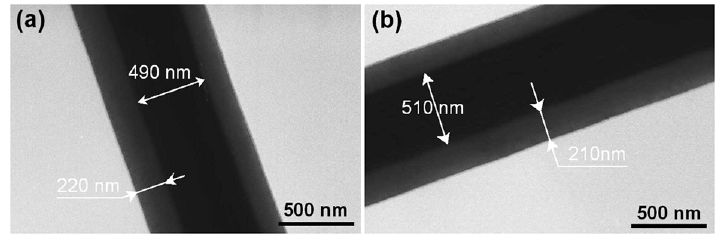
Natsathaporn et al (2023) electrospun silicone-based (polydimethylsiloxane, PDMS) fibrous membrane using a core-shell electrospinning method where the non-electrospinnable PDMS solution was fed through the core of the coaxial nozzle and the electrospinnable polyvinylpyrrolidone (PVP) or polyvinyl alcohol PVA) solution was the shell material. Both PVP and PVA were selected as the shell materials as they are immiscible with PDMS. A grounded heating plate set at 100 °C was used as the collector to initiate cross-linking of the PDMS core upon fiber deposition and subsequent heat treatment was carried out to cure the PDMS core. The electrospun PDMS/PVA core-shell fibers had a flattened shape, probably due to the low glass transition temperature of PVA which is lower than the curing temperature of 100 °C. PDMS/PVP core-shell fibers with PVP having a glass transition temperature at 172 °C maintained a round shape. After curing, the PVP shell was removed by washing the fibers in ethanol. Electrospun PDMS/PVP core-shell fibers have a diameter of 2.05 µm and after removal of PVP, the fibers have an average diameter of 1.52 µm. Calculated PVP:PDMS weight ratio from thermogravimetric analysis (TGA) showed no loss of PDMS during the electrospinning process. PVP was largely removed from the fibers with a small fraction detected after the washing process.
Electrospinning core-shell fibers with non-spinnable inorganic precursors as the core may offer some advantages over blending of the precursor with sacrificial polymer. With polymer blended inorganic precursors, sintering and subsequent pyrolysis of the polymer will give rise to more porous fibers. However when the precursor is electrospun as a single component in the core of a core-shell fiber, reduced porosity, decreased surface defects, uniform crystal packing of the sintered fiber can be expected. Dong et al (2024) demonstrated this using polyvinylpyrrolidone (PVP) as the shell material and precursors of TiO2 and ZrO2 as the core. Polycrystalline TiO2 fibers after sintering and pyrolysis of PVP showed excellent flexibility and a high Young's modulus of 54.3 MPa. Similarly, ZrO2 fibers using the same process showed Young's modulus and toughness of 130.5 MPa and 11.9 KJ/m3, respectively. Both TiO2 and ZrO2 produced using the core-shell electrospinning method showed significantly better mechanical properties compared to the conventional blended method.
Collagen is a natural extacellular matrix material and it is difficult to electrospin into fibers when it is dissolved in weak acid. While dissolving collagen in polar solvents have been successfully electrospun into fibers, there are controversy on the retention of its higher order molecular structure. Wakuda et al (2018) overcome this problem by using core-shell electrospinning to facilitate collagen fiber formation by dissolving collagen in acetate buffer solution to form the core and polyvinyl pyrrolidone (PVP) in acetate buffer solution as the shell. Unlike collagen, PVP readily forms fiber through electrospinning. This helps the formation of fibers with collagen at its core. PBS with 20% ethanol was used to wash away the PVP shell to leave behind pure collagen fibers which shows retention of higher order molecular structure.
Both coaxial and emulsion electrospinning can be used to form a linear core comprising of low molecular weight polymers and salt particles [Sun et al 2003].
With coaxial nozzle for electrospinning, core-shell fibers may also comprise of an electrospinnable core material and a non-electrospinnable shell material [Qian et al 2014]. An advantage of this system is that if the shell material solvent is of a higher boiling point and is also a solvent for the core-material, it will prevent the coagulation of the nozzle tip if a lower boiling point solvent is used for preparing the core-material. This technique has been used to electrospin superhydrophobic nanofiber by using electrospinnable polycaprolactone as the core and non-electrospinnable Teflon AF2400 as the shell material [Han and Steckl 2009]. In the construction of a flavored rapid oral drug release membrane, Ning et al (2011) used a mixture of non-electrospinnable sucralose and low molecular weight polyvinylpyrrolidone (PVP) as the shell and the drug diclofenac sodium (DS) and high molecular weight PVP as the core. The use of low molecular weight PVP in the shell ensures that the shell material will rapidly disintegrate in water to release the sucralose and its presence also helps to maintain an amorphous state of sucralose after electrospinning. Since DS is not electrospinnable, an electrospinnable, high molecular weight PVP was used as the core material. The high molecular weight PVP solution in the core was able to drive the electrospinning process while the low molecular weight PVP and sucralose solution coats over the core material.
Individual coated fiber
For some applications, it is desirable for the electrospun fiber to be coated with another material. This is possible using a coaxial electrospinning setup. With injectable electrospun membrane, the membrane is usually dipped in a hydrogel material such that the whole membrane is encapsulated within the hydrogel. Amagat et al (2023) used coaxial electrospinning to construct nanofibers that were individually coated with hydrogel to form a core-shell fiber. The core of the fiber was made of mechanically stronger poly(lactide-co-ε-caprolactone) (PLCL) and methacrylated gelatin (GelMA)/alginate hydrogel as the shell. Active ingredients were added to the hydrogel shell as required. The membrane with triangle and square-shaped mesh sized 0.72 or 1 cm2, respectively, was shown to support injection through inhalation and injection steps connected to a glass tube (inner diameter 0.9 mm). Similarly, the membrane can be ejected and inhalated back into a 20 G needle.
A core-shell structure has also been tested to reduce the degradation rate of certain polymers. Polyglycolic acid (PGA) exhibits good mechanical strength but its rapid degradation in fluid makes it unsuitable for use as an implantable scaffold. To reduce the degradation rate of PGA, Zhang et al (2024) used coaxial electrospinning to form a silk fibroin (SF) protective shell over PGA core. Electrospun scaffolds made of SF fibers, PGA fibers and PGA/SF core-shell fibers were immersed in PBS solution. The PGA fibers degraded into fragments at just 4 weeks while SF fibers remained physically unchanged for 16 weeks. PGA/SF core-shell fibers remained relatively unchanged up to 8 weeks but cracks on the SF shell caused the fibers to expand thereafter. Weight loss for PGA/SF scaffold was about 22% in the first 8 weeks but slowed down to about 25% weight loss at 16 weeks compared to complete degradation of PGA scaffold after 4 weeks and negligible weight loss of SF scaffold at 16 weeks.
Mechanical and physical properties
A core-shell fiber allows a stronger or stiffer material to support a weaker material. Schmidt et al (2025) constructed an electrospun core-shell fibrous membrane with the fiber having a polystyrene core and surface made of hydrophilic random copolymer of N-isopropylacrylamide (NIPAM) with N,N-(2-(diethylamino)ethyl)acrylamide (DEAAm) and N-(4-benzoylphenyl)acrylamide (BPAA), denoted as PIDB with the beads made of PIDB. With electrospun pure PIDB fibrous membrane, there was significant swelling of the fibers leading to shrinkage of pores and reduction in flux when the reactant solution was passed through it. However, the BOS PS/PIDB fibrous membrane showed less swelling and very low-pressure drop comparable to the neat PS nonwoven. The stiffness of the PS core may have prevented excessive swelling of the PIDB shell layer.
Active Compound Release
Drugs and other compounds are often loaded into nanofibers by blending them into the solution for electrospinning. However, compounds incorporated in this manner often exhibit burst release which may not be appropriate for some medical treatment and it is also not possible to control the release rate due to the even distribution of drugs throughout the fibers. Core-shell fibers with the active compounds loaded at the core have the advantage of having a slower release rate and the rate of release may be controlled by varying the thickness of the shell material. Yan et al (2014) varied the feed ratio between the inner polyvinyl alcohol solution and the outer chitosan solution and showed the reduction in the release of doxorubicin (DOX) from the core when the feed ratio of chitosan solution increases. Chitosan was selectively crosslinked by glutaraldehyde vapor to reduce DOX release rate. Although human ovary cancer cells (SKOV3) seeded on the drug loaded scaffold showed good attachment, proliferation and spreading initially, they start to deteriorate after 8 days which is due to the time dependent drug release from the fibers. Ding et al (2020) constructed a core-shell Eudragit S100 (ES100) fibers using triaxial nozzle electrospinning for colon-targeted release of aspirin. Both the core and shell of the fibers were made of ES100 but aspirin is only loaded into the core of the fiber. The presence of the outer ES100 wall provides an additional barrier for the inner aspirin to cross over before it is being released. When compared with homogeneously distributed aspirin in ES100 electrospun fibers, the core-shell fibers release a lower dose of aspirin in the first 2 hours against the former. This may offer greater protection of the stomach membrane with less aspirin being released until the drug loaded fibers reach the colon. There is a longer sustained release of aspirin for core-shell fibers with 50% and 95% drug release at 4.15 and 6.09 h respectively. This compared well to uniformly distributed drugs in the fiber with 50% and 95% drug release at 2.66 and 3.91 h respectively.
Successful demonstration of drug release has also been demonstrated by varying feed rate of either inner material [Joung et al 2011] or outer material. Active compounds have also been incorporated into core-shell fibers for other applications. Fertilizers have been shown to exhibit a controlled release profile over a month long period when they were loaded in the core with a biodegradable polymer shell matrix [Kampeerapappun and Phanomkate 2013].
Unique composite material
A fiber with differentiated materials between the core and the shell enables the creation of smart fibers where both materials work in unison in response to external stimulus. Core-shell fiber morphology has been shown to improve the shape memory response of some materials. With polycaprolactone-based shape memory polyurethane (CLSMPU), Zhuo et al (2011) found that encapsulating the material with a shell material of pyridine containing polyurethane (PySMPU) significantly improves the shape memory effect with good fixity and the added benefit of excellent anti-bacterial properties. With CLSMPU alone, the shape fixity was less than 80% but this increases to above 95% when PySMPU was used as the shell material.
Electrospun fibers offer several advantages in the introduction of self-healing property. Self-healing material generally derived this property through encapsulation of curable components within its structure. Damaged to the material will release the repair agents and initiate the self-healing process. Small diameter of electrospun fibers allow uniform coverage over the material to be protected with minimal influence on the bulk material properties. Any release of the repair agent will also be localized and with greater precision on the dosage as the agents come in small packages. Park et al (2010) created a self-healing coating using core-shell electrospinning where the two parts siloxanes were separately encapsulated within poly(vinylpyrrolidone) (PVP) shell for deposition on the substrate. The electrospun core-shell fiber mixtures were then embedded within a polyurethane coating. Successful passivation of the substrate from the environment was demonstrated when the polyurethane surface was scratched.
Electrospun core-shell fiber is also applicable to inorganic materials. Liu et al (2011) used this technique to create a unique core-shell carbon nanofiber with a hard outer shell and soft inner core for use as an anode material for lithium-based batteries. For electrospinning, the core material was made out of mineral oil while the sheath material was made from polyacrylonitrile. Post-electrospinning, the resultant fibers were carbonized to create this unique composition.
Zeng et al (2021) constructed a core-shell silicon (Si) and carbon (C) fiber using electrospinning for lithium-ion batteries application. Using a coaxial nozzle, a dispersion of Si nanoparticles in poly(methyl methacrylate) (PMMA) solution was injected through the core and the outer solution was a polyvinylpyrrolidone (PVP) solution. Heat treatment followed by carbonization at 800 °C was carried on the electrospun core-shell fibers to convert the PVP into carbon. The resultant Si@C fibers showed a smooth outer carbon layer with evenly dispersed Si nanoparticles at the core. This Si@C core-shell fiber membrane was shown to exhibit much better charge capacity compared to a Si/C blended fiber membrane.
Differentiated Functional Properties
Given that a core-shell fiber is made out of two independent materials, each material may impart a unique and independent functionality to the composite fiber. In biomedical application, a core-shell fiber may be made out of a core with a higher mechanical strength such as polyurethane and a shell material that encourages shell adhesion and proliferation such as collagen [Chen et al 2010]. In ultra-fast drug delivery system for oral application, Yu et al (2011) incorporated sucralose as a sweetener in the shell material while the core contains a poorly water-soluble drug. This combines several advantages of core-shell nanofiber such as incorporation of non-electrospinnable drugs, controlled drug release and differentiation of functional additives. Some polymers are prone to shrinking in water. Poly(lactic-co-glycolic acid) (PLGA) is a biodegradable and biocompatible polymer that makes it suitable in various tissue engineering applications. However, electrospun PLGA membranes exhibit significant shrinkage in water and PBS. Nagiah et al (2020) constructed an electrospun triaxial fiber with PLGA at the sheath layer and polycaprolactone (PCL) as the core fiber layer. PCL has good dimensional stability in water and PBS and will provide the necessary support to resist shrinkage. When tested in cell culture media, uniaxial PLGA (50:50) electrospun fibrous scaffold shrunk nearly 80% of its original dimension. However, the triaxial electrospun fibrous scaffold with PLGA at the sheath layer and polycaprolactone (PCL) as the core fiber layer did not show any significant shrinkage. The reduction in the shrinkage of triaxial electrospun fibrous scaffold probably allowed a higher number of cells to adhere onto it compared to uniaxial PLGA (50:50) electrospun fibrous scaffold and hence contributed to significantly level of cell activity detected in the triaxial electrospun fibrous scaffold.
Reduction of physical defects in high additives loaded fibers
Incorporation of compounds and additives through blending often limits the amount that can be added before defects such as beads are formed on the electrospun fibers. Electrospinning to form core-shell fibers may maintain the smooth outer surface of the fiber while allowing higher percentage of loading at the core of the fiber. Lisunova et al (2010) demonstrated this advantage with the electrospinning of fibers with high loading concentration of carbon nanotubes (CNT). 10 wt.% of polyacrylonitrile (PAN) in dimethylformamide (DMF) with 15 wt.% of CNTs produces electrospun fiber with visible physical defects but 15 wt% of cellulose acetate (CA) in dimethyl-acetamide (DMAc)/acetone (2:1) and 10 wt.% of PAN in DMF with 35 wt.% of CNTs produces smooth fibers. Although the concentration of CNTs per unit mass of the matrix material may be similar between blended fiber and core-shell fiber, the core-shell fiber was able to have a region of significantly higher CNT density.
Immiscible Liquid Electrospinning
Core-shell fibers can be electrospun using a single nozzle by mixing two solutions that forms separate layers. In an experiment by Bazilevsky et al (2007), the lighter polyacrylonitrile/dimetylformamide solution floats on top of the heavier poly(methyl methacrylate)/DMF solution. Core-shell electrospinning was initiated whenever a droplet of the heavier poly(methyl methacrylate) solution was sucked into the jet. Although the droplet may seems small, the volume is sufficient to draw over a meter of core-shell fibers. When the immiscible liquids are made into emulsions, linear arrangement of immiscible droplets along the core of the fiber was able to form core-shell fibers [Wang et al 2014, Li 2010].
Molecular self-organization
During electrospinning, molecules and ions in the solution may self-organized under the influence of the applied charges. Distribution and orientation of molecules and ions are influenced by several factors such as crystallinity, interaction between material mixture and molecular mobility. Tsaroom et al (2011) observed the formation of core-shell polymer-metal salt fibers with the positively charged metal salt concentrated at the core of the fibers after electrospinning with high positive charge. However, this was only seen when the metal salt is mixed with polyethylene oxide polymer but not with polyacrylic acid. It was hypothesized that the interaction between the negative ions of polyacrylic acid with the positive metal salt restricted any metal-salt ions distribution under the influence of the positive external charge. It is with polyethylene oxide, which is neutral, that core-shell structure was formed with metal salt rich core. However, application of negative high voltage does not see a concentration shift of the positive metal towards the shell. This has been attributed to crystallization of polyethylene oxide from the surface which prevented the aggregation of metal-salt at the surface. Xiong et al (2024) showed that with dopamine (DA) added to polyvinylidene fluoride (PVDF) and electrospun into fibers, the resultant PVDF/DA fiber showed a core-shell structure. The self-organization of the molecules has been attributed to the interactions between the -NH2 moiety in DA and -CF2 moiety in PVDF. The positive voltage applied for electrospinning compels the positively charged -NH2 moiety to migrate to the core of the electrospinning due to electrostatic repulsion. The distribution and orientation of the molecules due to dipolar interaction between -NH2 and -CF2 resulted in the formation of core-shell structure. This alignment of PVDF molecules may favor the formation of β-type structure of CH2-CF2.
Influencing fiber form
Helical inorganic fibers have been constructed by sintering core-shell fibers with the pure inorganic precursors as the core. This was demonstrated by Dong et al (2024) using polyvinylpyrrolidone (PVP) as the shell material and precursors of TiO2 and ZrO2 as the core. The formation of the helical fiber has been attributed to crack formation on the surface of one side of the initial core-shell fiber during sintering. During electrospinning, stiffness of the core alkoxide sol as it vaporizes led to an uneven shrinkage of the ductile shell due to the constraint from the core-shell interface and the surface of the fiber. This resulted in cracks on the fiber shell, which distribute asymmetrically about the fiber axis and rotate along the change of the curving direction. As the fiber shrinks due to the surface PVP decomposition, the fiber starts to curl towards the side with the crack. With a thinner shell, the uneven shrinkage of the shell from the core-shell interface and its surface was less pronounced and no coiling of the fibers were observed.
Challenges in core-shell electrospinning
One of the greatest challenges in core-shell electrospinning is to ability to get consistent core-shell morphology throughout the length of the fiber. Although reports on core-shell fiber often show transmission electron microscope (TEM) image of core-shell material distribution, it only represents a segment of it and segments without core-shell distribution may have been omitted. Continuous core-shell morphology requires stable and steady injection of the core and the shell solution during electrospinning and it is impossible to verify that there is no disruption to the core solution supply during the spinning process. However, in applications such as drug delivery, a non-continuous core-shell material distribution will not have any adverse impact on the drug delivery capability.
Research into the interaction between the core and shell fluid in a coaxial electrospinning setup has revealed some important requirements to facilitate smooth and continuous production of core-shell fibers. Vats et al (2021) did a comprehensive study in the effect of solvent miscibility between the core and shell solution on the production of core-shell fibers in a coaxial setup. Both extreme immiscibility and miscibility between the core and shell solution are detrimental to producing core-shell fibers. In complete immiscible solutions, the relatively high interfacial tension between the core and shell solution will cause the core to break up as the core solution tries to minimize its surface area in contact with the shell solution by forming a sphere. On the other extreme, completely miscible core and shell solution will remove the interface and cause premature gelation in the Taylor cone as the solutions mixed during the electrospinning process. Therefore, an ideal core and shell solution pairing would be partial miscibility between the two solutions. Although both solutions may be partially miscible, the concentration difference at the tip of the nozzle may cause local interfacial tension variation, creating a solutal Marangoni effect and resulting in beaded fibers. To minimize Marangoni flows and facilitate the production of continuous core-shell fiber, a small amount of sheath solvent was recommended to be added to the core prior to spinning. This will reduce the interfacial tension variation as the core and shell interface quickly reaches an equilibrium.
Although there is no apparent good method to verify the continuity of core-shell material distribution in the fiber, there is a viable method for testing whether hollow fiber structure is continuous.
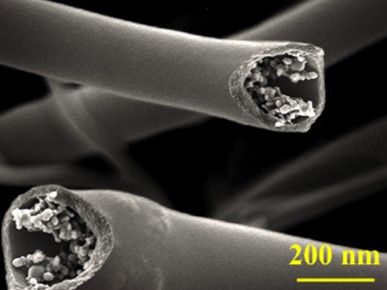
Application where a continuous hollow fiber structure is required may be check through the capillary action of the hollow fiber. With one end of the hollow fiber dipped in a fluid such as silicone oil and the other end left open in the air, wicking of the silicone oil through the hollow fiber structure will demonstrate that the fiber has a continuous hollow core. Confirmation of such continuous hollow fiber structure has been demonstrated by Na et al (2012) by electrospinning poly(vinylidene fluoride) (PVDF) shell and poly(vinyl alcohol) (PVA) core fibers. For this production, PVA solution was prepared using dimethyl sulfoxide(DMSO) - ethanol which ethanol is a non-solvent for PVDF. This causes precipitation of PVDF at the interface during electrospinning and subsequent gathering of materials around the interface thereby forming a hollow core.
Published date: 19 November 2014
Last updated: 13 January 2026
▼ Reference
-
Amagat J, Müller C A, Jensen B N, Xiong X, Su Y, Christensen N P, Friec A L, Dong M, Fang Y, Chen M. Injectable 2D flexible hydrogel sheets for optoelectrical/biochemical dual stimulation of neurons. Biomaterials Advances 2023; 146: 213284.
Open Access
-
Bazilevsky A, Yarin A L, Megaridis C M. Co-electrospinning of Core-Shell Fibers Using a Single-nozzle Technique. Langmuir 2007; 23: 2311.
-
Chang G, Zheng X, Chen R, Chen X, Chen L, Chen Z. Silver Nanoparticles Filling in TiO2 Hollow Nanofibers by Coaxial Electrospinning. Acta Phys-Chim Sin 2008; 24: 1790.
-
Chen R, Huang C, Ke Q, He C, Wang H, Mo X. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/collagen compound nanofibers for tissue engineering applications. Colloids and Surfaces B: Biointerfaces 2010; 79: 315.
-
Ding Y, Dou C, Chang S, Xie Z, Yu DG, Liu Y, Shao J. Core-Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020; 12: 2034.
Open Access
-
Dong S, Maciejewska B M, Schofield R M, Hawkins N, Siviour C R, Grobert N. Electrospinning Nonspinnable Sols to Ceramic Fibers and Springs. ACS Nano 2024; 18 (21): 13538.
https://pubs.acs.org/doi/full/10.1021/acsnano.3c12659Open Access
-
Han D, Steckl A J. Superhydrophobic and Oleophobic Fibers by Coaxial Electrospinning. Langmiur 2009; 25: 9454.
-
Joung Y K, Heo J H, Park J M, Park K D. Controlled Release of Growth Factors from Core-Shell Structured PLGA Microfibers for Tissue Engineering. Biomaterials Research 2011; 15: 78. http://www.ksbm.or.kr/pub/78-84.pdf.
-
Kampeerapappun P, Phanomkate N. Slow Release Fertilizer from Core-Shell Electrospun Fibers. Chiang Mai J Sci 2013: 40: 775.
Open Access
-
Kriel H, Sanderson R D, Smit E. Coaxial Electrospinning of Miscible PLLA-Core and PDLLA-Shell Solutions and Indirect Visualisation of the Core-Shell Fibres Obtained. Fibers & Textiles in Eastern Europe 2012; 20: 28.
Open Access
-
Li Q, Xu L, Hu C, Zhang Z, Yang D, Chen W, Williams G, Parker GJM, Gao F, Zhou F-L. Direct Jet Co-Electrospinning of Spinal Cord-Mimicking Phantom for Diffusion Magnetic Resonance Imaging. Coatings. 2024; 14(5):520.
https://www.mdpi.com/2079-6412/14/5/520 Open Access
-
Li Y. Emulsion-electrospinning of nanocrystalline cellulose reinforced nanocomposite fibres. Master of Applied Science Thesis 2010 The University of British Colombia.
Open Access
-
Lisunova M, Hildmann A, Hatting B, Datsyuk V, Reich S. Nanofibres of CA/PAN with high amount of carbon nanotubes by core-shell electrospinning. Composite Science and Technology 2010; 70: 1584.
-
Liu B X, Yu Y H, Chang J, Yang X J, Wu D Z, Yang X P. An enhanced stable-structure core-shell coaxial carbon nanofiber web as a direct anode material for lithium-based batteries. Electrochemistry Communications 2011; 13: 558.
-
Liu Y, Chen X, Gao Y, Liu Y, Yu D, Liu P. Electrospun Core-Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules. 2022 Jul 29;12(8):1057.
Open Access
-
Na H, Chen P, Wong S C, Hague S, Li Q. Fabrication of PVDF/PVA microtubules by coaxial electrospinning. Polymer 2012; 53: 2736.
-
Nagiah N, Murdock C J, Bhattacharjee M, Nair L, Laurencin C T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Scientific Reports 2020; 10: 609.
Open Access
-
Natsathaporn P, Herwig G, Altenried S, Ren Q, Rossi R M, Crespy D, Itel F. Functional Fiber Membranes with Antibacterial Properties for Face Masks. Adv. Fiber Mater. 2023; 5: 1519.
Open Access
-
Ning T, Zhou Y, Xu H, Guo S, Wang K, Yu D-G. Orodispersible Membranes from a Modified Coaxial Electrospinning for Fast Dissolution of Diclofenac Sodium. Membranes. 2021; 11(11):802.
Open Access
-
Park J H, Braun P V. Coaxial Electrospinning of Self-Healing Coatings. Adv. Mater. 2010; 22: 496.
-
Qian W, Yu D G, Li Y, Liao Y Z, Wang X, Wang L. Dual Drug Release Electrospun Core-Shell Nanofibers with Tunable Dose in the Second Phase. Int. J. Mol. Sci. 2014; 15: 774.
-
Reise M, Kranz S, Guellmar A, Wyrwa R, Rosenbaum T, Weisser J, Jurke A, Schnabelrauch M, Heyder M, Watts D C, Sigusch B W. Coaxial electrospun nanofibers as drug delivery system for local treatment of periodontitis. Dental Materials 2023; 39: 132.
Open Access
-
Schmidt M, Riecken C, Zussman E, Agarwal S, Schmalz H, Greiner A. Regio-Selective Functionalisation of Electrospun Materials at the Microscale. Advanced Materials Interfaces 2025; 12: 2400666.
https://advanced.onlinelibrary.wiley.com/doi/10.1002/admi.202400666 Open Access
-
Srivastava Y, Loscertales I, Marquez M, Thorsen T. Electrospinning of hollow and core/sheath nanofibers using a microfluidic manifold. Microfluid Nanofluid 2008; 4: 245.
-
Sun Z C, Zussman E, Yarin A L, Wendorff J H, Greiner A. Compound Core-Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003; 15: 1929.
-
Tsaroom A, Matyjaszewski K, Silverstein M S. Spontaneous core-sheath formation in electrospun nanofibers. Polymer 2011; 52: 2869.
-
Vats S, Anyfantakis M, Honaker L W, Basoli F, Lagerwall J P F. Stable Electrospinning of Core-Functionalized Coaxial Fibers Enabled by the Minimum-Energy Interface Given by Partial Core-Sheath Miscibility. Langmuir 2021; 37: 13265.
Open Access
-
Wang J, Yu Y, Gu L, Wang C, Tang K, Maier J. Highly reversible lithium storage in Si (core)-hollow carbon nanofibers (sheath) nanocomposites. Nanoscale 2013; 5: 2647.
-
Wang W, Wang L, Wang M. Evolution of core-shell structure: From emulsions to ultrafine emulsion electrospun fibers. Materials Letters 2014, article in press.
-
Wakuda Y, Nishimoto S, Suye S I, Fujita S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Scientific Reports 2018; 8: 6248.
Open Access
-
Xiong J, Wang L, Liang F, Mengying Li, Yabuta Y, Iqbal M A, Mayakrishnan G, Shi J, Kim I S. Flexible Piezoelectric Sensor Based on Two-Dimensional Topological Network of PVDF/DA Composite Nanofiber Membrane. Adv. Fiber Mater. 2024; 6: 1212.
https://link.springer.com/article/10.1007/s42765-024-00415-7Open Access.
-
Yan E, Fan Y, Sun Z, Gao J, Hao X, Pei S, Wang C, Sun L, Zhang D. Biocompatible core-shell electrospun nanofibers as potential application for chemotherapy against ovary cancer. Materials Science and Engineering C 2014; 41: 217.
-
Yu D G, Zhu L M, Branford-White C J, Yang J H, Wang X, Li Y, Qian W. Solid dispersions in the form of electrospun core-sheath nanofibers. International Journal of Nanomedicine 2011; 6: 3271.
Open Access
-
Yu W, Ma Q, Li X, Dong X, Wang J, Liu G. One-pot coaxial electrospinning fabrication and properties of magnetic-luminescent bifunctional flexible hollow nanofibers. Materials Letters 2014; 120: 126.
-
Zeng L, Xi H, Liu X, Zhang C. Coaxial Electrospinning Construction Si@C Core-Shell Nanofibers for Advanced Flexible Lithium-Ion Batteries. Nanomaterials. 2021; 11(12):3454
Open Access
-
Zhang Y, Rutledge G C. Electrical Conductivity of Electrospun Polyaniline and Polyaniline-Blend Fibers and Mats. Macromolecules 2012; 45: 4238.
-
Zhang Y, Jian Y, Jiang X, Li X, Wu X, Zhong J, Jia X, Li Q, Wang X, Zhao K, Yao Y. Stepwise degradable PGA-SF core-shell electrospinning scaffold with superior tenacity in wetting regime for promoting bone regeneration. Materials Today Bio 2024; 26: 101023.
Open Access
-
Zhuo H T, Hu J L, Chen S J. Coaxial electrospun polyurethane core-shell nanofibers for shape memory and antibacterial nanomaterials. eXPRESS Polymer Letters 2011; 5: 182.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech