Core-shell fibers are often used for molecular release or for the creation of composite fibers. In controlled drug release, the drug is often encapsulated within the core of the fiber such that the polymeric shell layer acts as a barrier to reduce unwanted burst release. Core-shell fibers may also be used to encapsulate monomers or initiators in self-healing materials. A common use of core-shell fibers composite is to have a mechanically stronger core material and a functional shell material.
A co-axial nozzle is the earliest method used in the production of core-shell fibers. However, other methods have since been found to be capable of forming core-shell fibers. In some solution mixtures, the molecules may self-organize during electrospinning. The carrier solvent for the molecules may be immiscible such that the solvents may separate and dry core-shell fibers are collected.
Co-axial nozzle
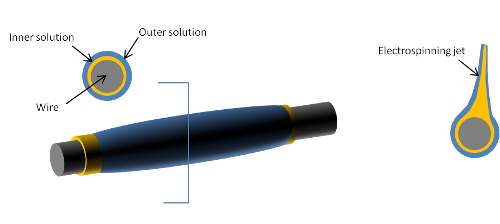
Core-shell fibers are most often produced by using a co-axial nozzle for electrospinning [Yarin et al 2007]. The co-axial nozzle comprises of two cylinders with a one cylinder situated within the core of a larger bore cylinder. Two different solutions are dispensed simultaneously through the inner and outer cylinders and charged in the same way as conventional single bore nozzle. There are several parameters that can be modified to control the size of the fibers and the volume ratio of the core and shell material. Fiber diameter can be controlled by the nozzle diameter while the volume ratio is by the feed-rate of the core and shell solution [Chakraborty et al 2009]. There are also some selection criteria for the core and shell solution pair.
When the surface tension of the core solution is high, there is an interfacial tension between the core and sheath polymer solutions and this reduces the miscibility between the two solutions. Qavi et al (2023) found that with lower concentration of polyethylene oxide (PEO) core solution the interfacial tension with the polycaprolactone (PCL) sheath solution was weak. This resulted in incomplete encapsulation of the core polymer by the sheath polymer. Another factor affecting core-shell fiber formation is the viscosity ratio between the core and sheath solution [Qavi et al 2023]. When the sheath solution viscosity is lower than the core solution viscosity, the sheath solution may not form a continuous wall around the core polymer. In contrast, when the sheath solution viscosity is higher, the increased viscous drag by the sheath solution encourages full encapsulation of the core solution.
Chakraborty et al (2009) recommended selecting solutions pair with similar volatility and use of intermediate solvent where miscibility of core and shell solution is poor.
However, this depends on the application. Some investigators have used the same polymer solution for the core and the shell for the purpose of controlling drug release from the core of the fiber. Although it may be more challenging to use imaging to view the core-shell structure, comparison of the drug release rate between core-shell fiber and fiber with uniformly dispersed drug showed the shell was able to retard the release of drug from its core [Ding et al 2020].
In coaxial electrospinning, the charges on the core and shell solution may be different although the voltage applied is the same. Bazrafshan et al (2018) found that in their coaxial electrospinning of collagen-g-poly(MMA-co-EA) (CME) shell and nylon 6 (N6) core. Individually, CME solution require a lower voltage for electrospinning jet initiation compared to N6 solution. In coaxial electrospinning of both solution, a lower applied voltage resulted in more CME fibers deposited with depositing fibers climbing towards the nozzle as if it was electrospinning CME only solution. At higher voltage, a 2D membrane was formed and this behaviour matches that of when N6 only solution was electrospun. Colored dyes in both solution in the coaxial setup showed CME coloration on the collected fibers at lower voltage and N6 coloration at higher voltage. Comparison of both solution properties showed similar surface tension and viscosity but N6 solution has a much higher conductivity than CME solution. At higher voltage, the core N6 solution may had broken free from the shell CME layer as shown by the bimodal fiber diameter distribution.
The formation of a continuous core-shell profile along the length of the electrospun fiber may depend on how smooth the electrospinning process is. If the Taylor cone at the tip of the nozzle is unstable, there will be agitation of the core and shell solution and the core-shell structure will be disrupted. Wu et al (2023) showed in their electrospinning of poly(L-lactide-co-ε-caprolactone) (PLCL) core/ collagen (Col) shell fibers using a coaxial nozzle that that without the addition of surfactant (Tween 80), the Taylor cone is unstable with several jets erupting from it. The collected fibers were beaded with no distinct core-shell structure. In contrast, when Tween 80 was added to both PLCL and col solution for electrospinning, the Taylor cone was stable and distinct core-shell fibers were formed.
Core-shell fibers produced by coaxial nozzle electrospinning typically have a larger diameter compared to uniaxial nozzle electrospinning. This may be attributed to the greater amount of solution extruded from the coaxial nozzle. However, it is possible to reduce the diameter of the core-shell fiber by modifying the nozzle. Ding et al (2020) constructed a core-shell Eudragit S100 (ES100) fibers using triaxial nozzle electrospinning with the outermost layer extruding only pure solvent and both the core and second layer extruding ES100 polymer solution. Uniaxial nozzle electrospinning of ES100 polymer solution gave an average diameter of 870 nm. However, the triaxial nozzle electrospun ES100 polymer solution had an average diameter of 740 nm. Although the cumulative fluid flow rate from the triaxial nozzle was larger than the uniaxial electrospinning solution flow rate, the former produced fiber with smaller diameter. Hence, the presence of the outer solvent mixture was able to delay the solidification of the electrospinning jet while allowing the polymer solution to stretch and thin further.
Immiscible solutions
Having two immiscible solutions for electrospinning has been shown to produce core-shell fibers. This has been shown by Bazilesky et al (2007) where the lighter polyacrylonitrile/dimetylformamide solution floats on top of the heavier poly(methyl methacrylate)/DMF solution. Core-shell electrospinning was initiated whenever a droplet of the heavier poly(methyl methacrylate) solution was sucked into the jet. Although the droplet may seems small, the volume is sufficient to draw over a meter of core-shell fibers. As an evolution of this process, by passing a wire through two immiscible solutions, the wire is coated with two layers of solution [Forward et al 2012]. At sufficient voltage applied to the solution, electrospinning is initiated and a core-sheath fiber structure are formed.
When the immiscible liquids are made into emulsions, linear arrangement of immiscible droplets along the core of the fiber was able to form core-shell fibers [Wang et al 2014, Li 2010]. A possible combination for emulsion electrospinning is a water-in-oil emulsion with a surfactant to form the immiscible droplets [Wang et al 2014]. Emulsion electrospinning is of particular interest in the encapsulation of drugs as it allows water soluble drugs to be encapsulated within a polymeric shell for controlled drug release.
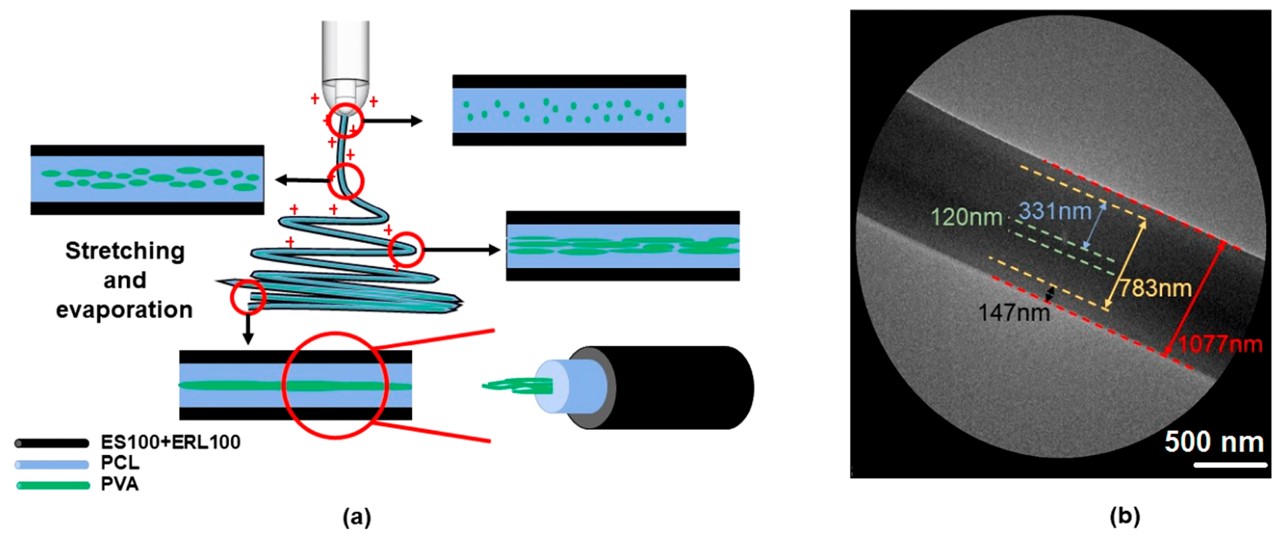
It is hypothesised that in the emulsion mixture of oil/solvent and water, the oil/solvent evaporates faster than the water during electrospinning. The movement of the oil/solvent to the surface isolates the aqueous phase in the core and induces demulsification. This movement of the liquids together with the solutes they are carrying formed a core-shell fiber after all the solvent and water has evaporated. Taking advantage of these movements, Wang et al (2023) electrospun a tri-layered core-shell fiber with the model drug metronidazole (MTD) at its core. A co-axial nozzle was used and Eudragit polymers were feed through the outer ring while the core was the emulsion solution of MTD, poly(ε-caprolactone) (PCL) as the oil phase, poly(vinyl alcohol) (PVA) as the water phase, and Pluronic F-127 (PF-127, a nonionic surfactant) as an emulsifier. The resultant tri-layer fiber was made of PVA/MTD at the core with diameter of about 120 nm, a middle layer of PCL/MTD with thickness of about 331 nm and the surface Eudragit layer with thickness of about 147 nm hence bringing the total diameter of the fiber to about 1077 nm.
Self-Organization
Distribution and orientation of molecules and ions are influenced by several factors such as crystallinity, interaction between material mixture and molecular mobility. Tsaroom et al (2011) observed the formation of core-shell polymer-metal salt fibers with the positively charged metal salt concentrated at the core of the fibers after electrospinning with high positive charge. However, this was only seen when the metal salt is mixed with polyethylene oxide polymer but not with polyacrylic acid. It was hypothesized that the interaction between the negative ions of polyacrylic acid with the positive metal salt restricted any metal-salt ions distribution under the influence of the positive external charge. It is with polyethylene oxide, which is neutral, that core-shell structure was formed with metal salt rich core. However, application of negative high voltage does not see a concentration shift of the positive metal towards the shell. This has been attributed to crystallization of polyethylene oxide from the surface which prevented the aggregation of metal-salt at the surface.
Migration of ions to form electrospun core-shell structure have also been demonstrated in cations mixtures. Maafa et al (2021) constructed CdO/ZnO core/shell nanofibers by first electrospinning a precursor solution of poly(vinyl alcohol), zinc acetate dihydrate, and cadmium acetate dihydrate. A core-shell distribution of CdO and ZnO was observed after calcination at 400°C with Cd-rich core and Zn-rich shell. Maafa et al (2021) suggest that this distribution was due to PVA and Zn2+ coordination that brings Zn2+ to the surface when a charge is applied. Subsequently, temperature gradient between the shell and the core during calcination was hypothesised to facilitate diffusion of Cd2+ to the core.
Migration of ions during electrospinning may also be used with UV photopolymerization technique to produce stable core-shell fibers. Niu et al (2016) used this concept to produce core-shell fibers with polyvinyl pyrrolidone (PVP) as the core material and Si atoms as the shell layer. For their electrospinning, thiol-ene monomer with Si atom and the initiator were mixed into the PVP solution. During electrospinning, evaporation of the solvent brought the smaller thiol-ene monomer with Si atom and the initiator to the surface of the electrospinning jet. Under UV, the thiol-ene monomer with Si atom polymerizes on the surface of the electrospinning jet prior to deposition on the collector. Using similar concept of ion migration, Niu et al (2016b) added a photoinitiator containing fluorine into polyacrylonitrile (PAN) solution with dimethylformamide (DMF) as the solvent. During electrospinning, the photoinitiator migrates to the surface of the fiber. This resultant photosensitive fiber was used for initiating the polymerization of tripropylene glycol diacrylate (TPGDA) and hydroxyethyl acrylate (HEA) monomers via UV irradiation on the PAN fiber surface. Note that this reaction is carried out post electrospinning. Post reaction imaging using TEM showed a distinct core-shell fiber structure.
The flow of polymers with different molecular weight within the solution during electrospinning may also lead to separation of the molecules. Niu et al (2015) demonstrated this with by preparing precursor solution with a mixture of low-, middle- and high- molecular weight polyvinyl alcohol. During electrospinning, they hypothesized that the low-, middle- and high- molecular weight polyvinyl alcohol will separate to form three layers with the lowest molecular weight PVA at the core instead of uniform distribution. They supported this hypothesis by replacing low molecular weight PVA with polyvinyl pyrrolidone (PVP) for electrospinning and followed by the removal of PVP to give hollow tube.
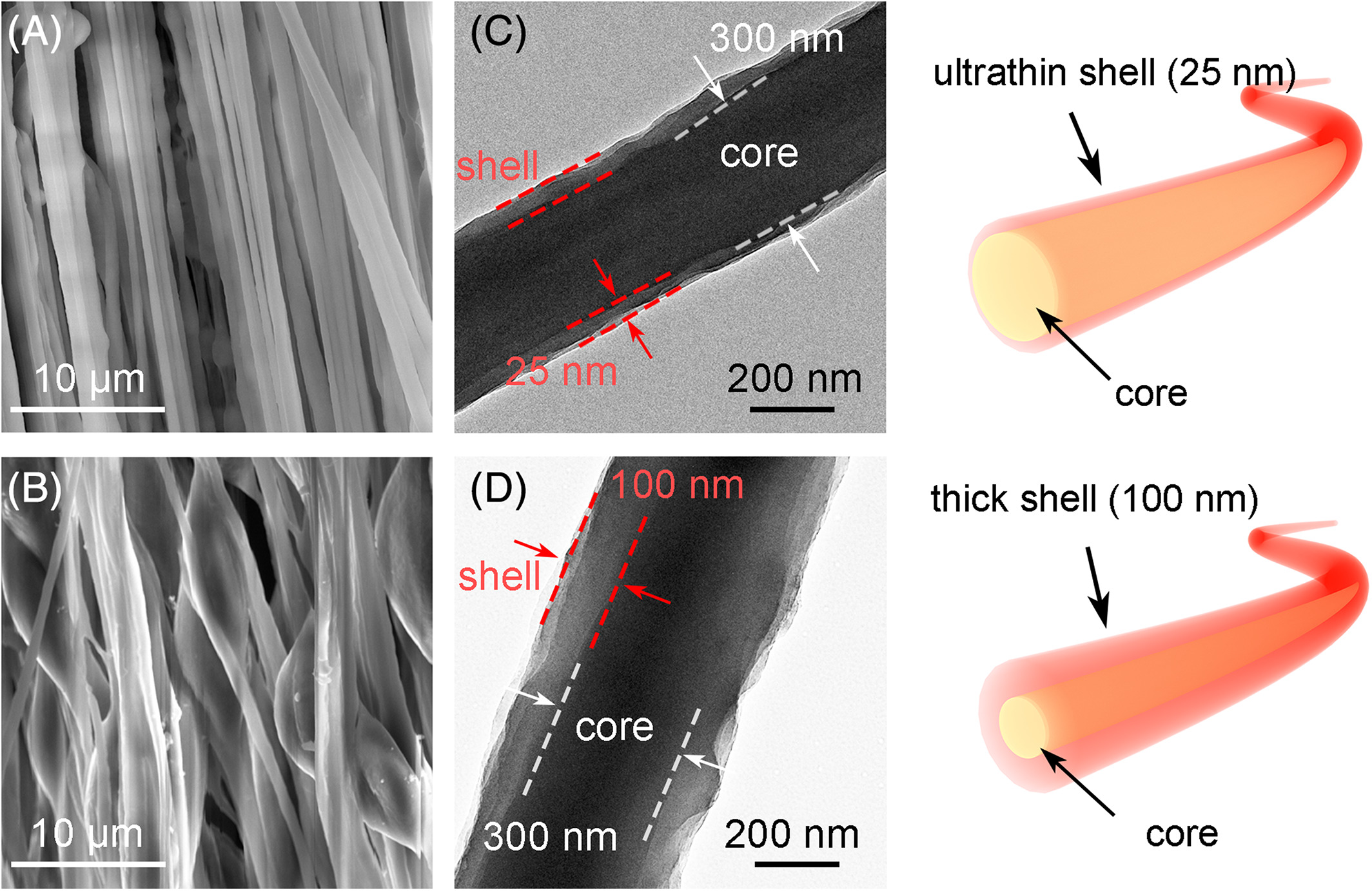
Liu et al (2019) showed that electrospinning a solution blend of poly(3,3'"-didodecyl quarter thiophene) (PQT-12) and poly(ethylene oxide) (PEO) was able to generate core-shell nanofiber with PEO as the core and PQT-12 as the shell. PQT-12 is a conjugated p-type semiconducting polymer which are difficult to electrospin into fibers on its own due to its high crystallinity and low molecular weight thus high molecular weight PEO is needed as a structural support to carry this molecule. During electrospinning, PQT-12 separated from PEO to form a thin layer on the surface. With 25 wt% PQT-12 in the PQT-12/PEO solution, the resultant fiber has a diameter of 350 nm and a shell of 25 nm. However, with higher ratio of PQT-12, beaded fibers start to form. This is probably due to insufficient PEO to provide structural base support for PQT-12.
Published date: 08 March 2016
Last updated: 04 June 2025
▼ Reference
-
Bazilevsky A, Yarin A L, Megaridis C M. Co-electrospinning of Core-Shell Fibers Using a Single-nozzle Technique. Langmuir 2007; 23: 2311.
-
Bazrafshan Z, Stylios G K. One-Step Fabrication of Three-Dimensional Fibrous Collagen-Based Macrostructure with High Water Uptake Capability by Coaxial Electrospinning. Nanomaterials 2018; 8(10): 803.
Open Access
-
Chakraborty S, Liao I C, Adler A, Leong K W. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv Drug Deliv Rev 2009; 61: 1043.
-
Ding Y, Dou C, Chang S, Xie Z, Yu DG, Liu Y, Shao J. Core-Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020; 12: 2034.
Open Access
-
Forward K M, Flores A, Rutledge G C. Coaxial-Free Surface Electrospinning. The Fiber Society 2012 Fall Meeting. Pp. 98
Open Access
-
Li Y. Emulsion-electrospinning of nanocrystalline cellulose reinforced nanocomposite fibres. Master of Applied Science Thesis 2010 The University of British Colombia.
Open Access
-
Liu D, Shi Q, Jin S, Shao Y, Huang J. Self-assembled core-shell structured organic nanofibers fabricated by single-nozzle electrospinning for highly sensitive ammonia sensors. Infomat 2019 Article in Press.
Open Access
-
Maafa I M, Al-Enizi A M, Abutaleb A, Zouli N I, Ubaidullah M, Shaikh S F, Al-Abdrabalnabi M A, Yousef A. One-pot preparation of CdO/ZnO core/shell nanofibers: An efficient photocatalyst. Alexandria Engineering 2021; 60: 1819.
Open Access
-
Niu C, Meng J, Wang X, Han C, Yan M, Zhao K, Xu X, Ren W, Zhao Y, Xu L, Zhang Q, Zhao D, Mai L. General synthesis of complex nanotubes by gradient electrospinning and controlled pyrolysis. Nature Communications 2015; 6: 7402.
Open Access
-
Niu Q, Zeng L W, Mu X Y, Nie J, Ma G P. Preparation and characterization of core-shell nanofibers by electrospinning combined with in situ UV photopolymerization. Journal of Industrial and Engineering Chemistry 2016 Article in press
-
Niu Q, Mu X, Nie J, Ma G. Potential fabrication of core-shell electrospun nanofibers from a two-step method: Electrospinning and photopolymerization. Journal of Industrial and Engineering Chemistry 2016b, Article in press.
-
Qavi I, Tan G. Process control of electrospinning artificial fenestrated capillary vessels. Materials & Design 2023 ; 227: 111708.
Open Access
-
Tsaroom A, Matyjaszewski K, Silverstein M S. Spontaneous core-sheath formation in electrospun nanofibers. Polymer 2011; 52: 2869.
-
Wang W, Wang L, Wang M. Evolution of core-shell structure: From emulsions to ultrafine emulsion electrospun fibers. Materials Letters 2014; 124: 192.
-
Wang Y, Liu L, Zhu Y, Wang L, Yu D-G, Liu L-y. Tri-Layer Core-Shell Fibers from Coaxial Electrospinning for a Modified Release of Metronidazole. Pharmaceutics. 2023; 15(11):2561.
https://www.mdpi.com/1999-4923/15/11/2561Open Access
-
Wu C, Wang H, Cao J. Tween-80 improves single/coaxial electrospinning of three-layered bioartificial blood vessel. J Mater Sci: Mater Med 2023; 34: 6.
Open Access
-
Yarin A L, Zussman E, Wendorff J H, Greiner A. Evolution of core-shell structure: From emulsions to ultrafine emulsion electrospun fibers. J. Mater. Chem. 2007; 17: 2585.
▲ Close list
 ElectrospinTech
ElectrospinTech