Natural extracellular matrix (ECM) is typically made out of collagen nanofibers and other biomolecules. In bone, the collagen nanofibers are covered with nanohydroxyapatite to give it greater structural strength. Numerous research have shown that bone related cells such as osteoblast [Fujihara et al 2005] and mesenchymal stem cells [Ekaputra et al 2009] grow well on electrospun nanofiber membrane. The versatility of electrospinning has allowed researchers to investigate various ways to use this process for bone healing and repair. In some cases, electrospun scaffold may be used directly as the bone repair scaffold. For others, electrospun fibers may be used with other implants to accelerate bone integration.
The basic form of electrospun output is a two dimensional membrane. As cells have been shown to adhere and proliferate well on electrospun two dimensional scaffold, early research on its application on bone was focused its utilization as a guided bone regeneration membrane. These membranes are usually used in calvarial defect, omentum and sterna injury which does not require thick scaffold. The electrospun membrane in this application typically functions as a guided bone regeneration graft which works as a barrier to prevent soft tissue invasion into the defect site [Dimitriou et al 2012].
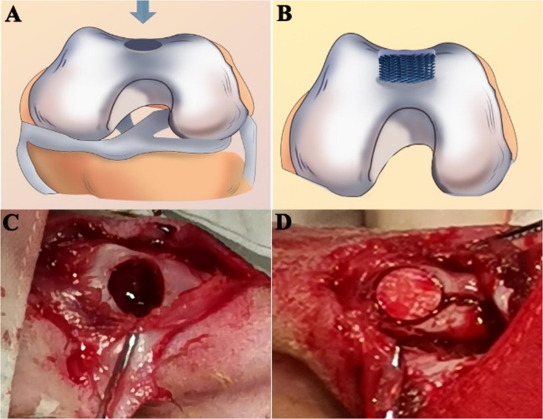
In cavarial defects repair, a scaffold with a minimum level of stiffness and strength is preferred especially if the defect is larger. Pensa et al (2019) constructed such a scaffold using electrospun poly-ε-caprolactone (PCL) /gelatin and 3D printed poly(lactic acid) (PLA) mesh. Instead of electrospinning and depositing PCL/gelatin fibers on 3D printed mesh, the PLA filaments were 3D printed on the electrospun fiber mesh. This has the advantage of creating a stronger bond between the 3D printed structure and the electrospun mesh. In 3D printing, the extruded filament in its semi-molten state will partially melt the PCL component in the electrospun fibers due to its lower melting point. An in vivo rat cranial defect study showed that the reinforced electrospun scaffolds with the 3D printed struts facing the outer surface of the skull did not elicit an immune or foreign body response after 20 weeks of implementation.
To reduce infection from the surgery, Jin et al (2018) incorporated Ag-CaP into electrospun chitosan (CS) fibers. Ag ion is known to be an effective antibacterial agent. The resultant electrospun Ag-CaP/CS exhibited a sustainable release of Ag+ and inhibited the adhesion and growth of Staphylococcus mutans. Its biocompatibility was shown by the adhesion and proliferation of bone marrow stromal cells cultured on it over 11 days. Cell viability was highest for CaP/CS electrospun scaffold. When there were Ag added (0.075% and 0.14%), the cell viability was reduced which showed that Ag+ does inhibit cell proliferation. However, with the added Ag, cell viability on Ag-CaP/CS electrospun scaffold was still better than pure electrospun CS scaffold. Liu et al (2022) constructed a multifunctional nanofibrous membrane using electrospun polylactic acid/hydroxyapatite (PLLA/HA) composite fiber as the base material. hydroxyapatite nanowires were blended into PLLA solution followed by electrospinning. This nanofibrous substrate material was then dipped in Sr2+, Cu2+ and Py in electrolyte solution for electrochemical deposition. The resultant fiber is a composite fiber made of PLLA/HA@SrHA/ Cu/PPy. Strontium was added to the fiber to improve osteoblast activity while copper ion is known to exhibit antibacterial properties and has been included in the composite fiber to reduce infections at the implant site. Polypyrrole was added as a regulator to encourage uniform distribution of Sr2+ and Cu2+ over the fiber surface. The resultant multifunctional PLLA/HA@SrHA/Cu/PPy composite fiber membrane showed close to 100% inhibition against Staphylococcus aureus and Escherichia coli bacteria. The PLLA/HA@SrHA/Cu/PPy composite fibers also showed good osteogenesis and angiogenesis properties through the culture of osteoblast and vascular endothelial cells (VEC) respectively.
The ease of introducing functional agents such as drugs and biomolecules to electrospun fibers meant that biochemical cues may be expressed by the resultant scaffold. Al-Baadani et al (2023) constructed a membrane with a mixture of two different drug loaded fibers, polycaprolactone (PCL) containing simvastatin (SIM) and gelatin fibers containing Substance P (SP) for bone regeneration. SP is known to enhance cell recruitment and this is essential for the initial healing while SIM is for revascularization and osteogenic induction. The release rate of SIM from PCL fibers was relatively slow with complete release at 31 days. This is probably due to the hydrophobic characteristic of PCL which slows the diffusion of SIM from the core to the surface. In contrast, more than 40% of SP was released from the gelatin fibers within the first day followed by more sustained and complete release at day 14. The rapid release of SP may be due to the hydrophilic nature of gelatin and its faster degradation. The initial rapid release of SP favors the recruitment and adhesion of MSCs while the sustained release of SIM encourages revascularization and osteogenesis. By having a mixture of these two drug-containing fibers, the cellular response at the surgical site can be managed leading to better bone regeneration outcome.
Stainless steel and titanium are the most commonly used material for bone or joint replacement due to their structural strength and biocompatibility. Researchers have been looking for ways to improve integration between the implants and host bone so that the implant does not become loose over time. Electrospun nanofibers have been tested as an implant integration coating. It is generally straightforward to deposit electrospun nanofibers on metal implants and this has been demonstrated on titanium implant [Ravichandran 2009] and magnesium alloy sheet [Soujanya et al 2014]. Ravichandran (2009) showed that adhesion of mesenchymal stem cells (MSC) on titanium plate and alloy is significantly enhanced with nanofiber coating even with man-made polymer such as poly(lactic acid)-co-poly(glycolic acid) (PLGA). Incorporation of the PLGA nanofiber with collagen and hydroxyapatite (HA) further increases the adhesion of MSCs on the implant surface. Titanium alloy coated with PLGA/collagen and HA nanofibers showed better proliferation and exhibited significantly greater alkaline phosphatase activity and mineral secretion than untreated titanium alloy.
While metals have been widely used in cortical bone repair, it suffers from stress shielding effect due to the much higher modulus of steel and to a lesser extent, titanium, compared to the bone. Scientists and researchers have been exploring alternative materials which have the strength and modulus closer to that of cortical bones. Jia et al (2021) constructed a polyether-ether-ketone/poly(methyl methacrylate)/carbon fiber (PEEK/PMMA/CF) ternary composites prepared by electrospinning and hot pressing which has mechanical properties similar to that of cortical bones. PEEK and PMMA fibers were separately prepared by electrospinning and crushed into short strands in anhydrous ethanol using a high-speed pulverizer. CF was added and homogenised with the crushed PEEK and PMMA fibers before hot pressing at a pressure of 250 MPa and a temperature of 160 °C in a mold. The PEEK/PMMA/CF ternary composite's Young's moduli (4.67 to 6.37 GPa), tensile strength (70 to 150 MPa) and bending strength (140 to 160 MPa) was similar or close to cortical bone. In vivo studies showed that the PEEK/PMMA/CF ternary composite exhibits good biocompatibility and no interference with X-rays, CT scan and MRI examinations.
Progress in electrospinning has increase the range of structures that can be produced using this process. Several methods have been developed for construction of 3D scaffold through electrospinning. Using the dynamic flow method, Teo et al was able to construct a 3D nanofibrous scaffold that is made out of yarn microstructure [Teo 2009, Teo et al 2007]. Alternate soaking mineralization (Calcium salt and phosphate salt solution) method was used to deposit nHA onto the yarn microstructure followed by freeze drying. Ngiam (2010) demonstrated the use of mineralized three-dimensional scaffold made out of nanofibrous yarns in a rabbit ulna defect. All the defects with filler scaffold showed approximately 50% new bone regeneration compared to about 38% for blank control after 3 months of implantation.
Enhancement in the fracture toughness with nanofibers interlayers between two different materials has been demonstrated in composites. This property may be used to improve the bonding between bone cement and metal implants. Khandaker et al (2014a, ) used electrospun polycaprolactone fibers (diameter range from 919 nm to 1.25 µm) as interlayers between implant and cement. In this case, the implant was made of titanium (Ti) and the cement was poly methyl methacrylate (PMMA). The effect of fiber patterns was tested and it was found that fracture toughness of the device where unidirectional fibers were used was much better than Ti/PMMA without fibers. However, with bi-directional fibers, the fracture toughness was less than Ti/PMMA without fibers [Khandaker et al 2014a]. In general, the presence of the fibers increases the surface roughness of the metal implant and this decreases the micromovement of cracks at the interface of Ti/PMMA [Khandaker et al 2014b].
Several in vitro studies have prove that electrospun nanofibers exhibit good biocompatibility, cell adhesion and proliferation for bone related cells. The electrospinning process has also been modified such that it can be used for various aspects of bone treatment. This may be bone implants, implant surface treatment or to enhance bonding strength.
Linder et al (2020) used a modified parallel electrodes collector electrospinning method to wrap highly aligned polycaprolactone (PCL) polymer nanofibers around individual 1393 bioactive glass microfibers to mimic the structure of osteon. The setup was made of two rotatable round plates and a stationary holder in the center of the plates to insert the glass microfiber between the plates. During electrospinning, fibers would deposit on the edges of the round plates. Rotation of the plates in opposing directions wraps the fibers around the center glass microfiber. Having electrospun fibers wrapped around the glass microfiber ensures that the scaffold remains intact even if the bioglass breaks. In vitro study showed the polymer wrapping did not inhibit the degradation of the bioglass for conversion into calcium phosphate. Further the aligned PCL fibers were shown to facilitate cell migration and elongation along the length of the scaffold. With this electrospinning setup, other biocompatible rods or cylinders may be coated with aligned nanofibers.
The versatility of this process will see more innovative ways to enhance bone treatment
The lack of mechanical stiffness and strength in scaffolds made purely of electrospun fibers limit its use as bone regenerative scaffolds. To overcome this inherent weakness while taking advantage of the benefits of nanofibers in cell proliferation and adhesion, Gonzalez-Pujana et al (2022) using a combination of 3D printing and electrospinning to construct a hierarchical polycaprolactone (PCL) scaffold for bone replacement. Fused deposition modeling (FDM) 3D printing method was used to produce a base made of square grids followed by electrospinning. Successive alternate 3D printing and electrospinning was carried out to build up the scaffold. With this, the 3D printed struts provide the required mechanical strength while the electrospun nanofibers sandwiched between the layers of struts provides a suitable surface for cell adhesion. Oxygen plasma treatment was carried out on both ends of the scaffold to promote cell attachment. Primary murine MC3T3-E1 preosteoblasts seeded in the scaffold were found to attach well on the nanofibers and migrated onto the struts by day 21. Over the 21 days of culture, the MC3T3-E1 cells showed mineralization, increased alkaline phosphatase (ALP) activity and upregulated expression of early and late osteogenic markers compared to the cells cultured on 2D culture plates. This demonstrated the potential use of this hierarchical structure as bone regenerative scaffold with osteogenic differentiation capability.
The periosteum is a layer of tissue that covers the bone. This tissue plays an important role in providing nourishment to the bone and facilitates osteogenesis. However, this tissue is often damaged when there are bone injuries. The presence of a periosteum scaffold may help to facilitate and accelerate bone healing. Tao et al (2020) attempted to create an artificial periosteum made of polycaprolactone (PCL)/carboxymethyl chitosan (CMCS)/sodium alginate (SA)-based micron-fibers fabricated by emulsion electrospinning. In this mixture of materials, PCL provides the mechanical strength, CMCS for biocompatibility and SA for improving the mechanical strength and stability of CMCS. As CMCS and SA dissolve in water while PCL dissolves in an organic solvent, an emulsifier is needed to mix them together. In this case, Span 80 was used for emulsification and the resultant solution has a milky white appearance. After electrospinning to form PCL/CMCS/SA microfibers membrane, cross-linking using CaCl2 ethanol solution to enhance the mechanical properties and stability of the membrane. Tests for early osteogenic related gene (Runx2, ALP) expression by osteoblast cells cultured on electrospun PCL/CMCS/SA microfibers membrane showed significantly higher expression compared to electrospun microfibers membrane made of PCL, PCL/CMCS and PCL/SA.
In other bone replacement scaffolds, there is a need to prevent fibroblast from migrating into the bone area. Liu et al (2021) used electrospinning and 3D printing to construct a hybrid bi-layer scaffold for the purpose of separating the soft tissue layer and the bone area. Polycaprolactone/gelatine (PCL/Gel) nanofibre membranes was first electrospun followed by conjugation with heparin. 3D printing of PCL/Gel/nano-hydroxyapatite (n-HA) scaffold was carried out over the PCL/Gel-heparin membrane to form a bi-layer scaffold. <>In vivo study on a rabbit osteochondral defect model showed that the hybrid bi-layer scaffold group showed a higher degree of new bone formation than the 3D printed PCL/Gel/n-HA scaffold and control group after 5 and 20 weeks. With the electrospun membrane positioned on the surface and the defect filled in by the 3D printed bulk volume, the membrane prevents surrounding fibrous connective tissues from migrating into the bone defect. The inner layer with the 3D printed macropores allows bone regeneration while supporting the surface membrane.
In tendon/ligament injuries, one challenge is the reintegration of the ruptured tendon or ligament to the bone. Electrospun membrane has been tested positively as guided bone regeneration graft [Dimitriou et al 2012] and the same concept may be applied for re-establishment of tendon/ligament to bone interface. Chen et al (2018) tested the effectiveness of electrospun random and dual-layer aligned-random silk fbroin poly(l-lactic acid-co-e-caprolactone) (P(LLA-CL)) nanofibrous scaffolds (ARS) in tendon-to-bone healing in a rabbit extra-articular model. Autologous Achilles tendon was wrapped either in ARS or electrospun random silk fibroin/P(LLA-CL) membrane and passed through the bone tunnel, while the control group was unwrapped Achilles tendon transplanted directly. Various parameters such as ultimate load-to-failure and stiffness, collagen maturity and new bone formation was better with electrospun membrane wrap and the ARS wrap demonstrating the best results in the 12 weeks study.
In cartilage and bone repair, the interface between the two needs to be considered. Not only are they inhibited by different cells, their functions are also different hence there is a need to direct regeneration in separate directions if a single scaffold is to be used. Mellor et al (2020) used multi-phasic 3D-bioplotting to construct the main body of the scaffold for cartilage and bone repair. In the part meant for cartilage regeneration, decellularized bovine cartilage extracellular matrix (dECM) hydrogel was injected to the 3D-bioplotted poly(ε-caprolactone) (PCL) scaffold. For bone regeneration, 20% β-tricalcium phosphate (TCP)/ 80% PCL was 3D-bioplotted. An electrospun PCL membrane layer was added between the two 3D-bioplotted scaffolds. This electrospun PCL membrane layer is needed to inhibit cell migration between the scaffolds layers. In a clinical setting, this layer will also prevent blood vessels from invading the chondrogenic portion of the scaffold. In vitro study using human adipose-derived stem cells (hASC) showed that the electrospun membrane was able to effectively separate the cell populations while evidence of site-specific hASC osteogenesis and chondrogenesis was observed.
Published date: 16 October 2018
Last updated: 14 May 2024
▼ Reference
-
Al-Baadani M A, Xu L, Cai K, Yie K H R, Shen Y, Al-Bishari A M, Al-Shaaobi B A, Ma P, Shen X, Liu J. Preparation of co-electrospinning membrane loaded with simvastatin and substance P to accelerate bone regeneration by promoting cell homing, angiogenesis and osteogenesis. Materials Today Bio 2023; 21: 100692.
Open Access
-
Cai J, Wang J, Ye K, Li D, Ai C, Sheng D, Jin W, Liu X, Zhi Y, Jiang J, Chen J, Mo X, Chen S. Dual-layer aligned-random nanofibrous scaffolds for improving gradient microstructure of tendon-to-bone healing in a rabbit extra-articular model. International Journal of Nanomedicine 2018;13: 3481
Open Access
-
Dimitriou R, Mataliotakis G I, Calori G M, Giannoudis P V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Medicine 2012; 10: 81.
Open Access
-
Ekaputra A K, Zhou Y, Cool S M, Hutmacher D W. Composite Electrospun Scaffolds for Engineering Tubular Bone Grafts. Tissue Engineering 2009; 15: 3779.
-
Fujihara K, Kotaki M, Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005; 26: 4139.
-
Jia W, Cui D, Liu Y, Ji X, Sun M, Cheng Z, Luo Y, Liu G. Polyether-ether-ketone/poly(methyl methacrylate)/carbon fiber ternary composites prepared by electrospinning and hot pressing for bone implant applications. Materials & Design 2021; 209: 109893.
Open Access
-
Gonzalez-Pujana A, Carranza T, Santos-Vizcaino E, Igartua M, Guerrero P, Hernandez RM, de la Caba K. Hybrid 3D Printed and Electrospun Multi-Scale Hierarchical Polycaprolactone Scaffolds to Induce Bone Differentiation. Pharmaceutics. 2022; 14(12):2843.
Open Access
-
Jin S, Li J, Wang J, Jiang J, Zuo Y, Li Y, Yang F. Electrospun silver ion-loaded calcium phosphate/chitosan antibacterial composite fibrous membranes for guided bone regeneration. International Journal of Nanomedicine 2018:13 4591.
Open Access
-
Khandaker M, KC U, Khadaka A. Effect of fiber patterns on the fracture of implant/cement interfaces. Procedia Engineering 2014a; 90: 32.
-
Khandaker M, Utsaha K C, Morris T. Fracture toughness of titanium-cement interfaces: effects of fibers and loading angles. International Journal of Nanomedicine 2014; 9: 1689.
Open Access
-
Linder H R, Glass A A, Day D E, Sell s A. Manipulating Air-Gap Electrospinning to Create Aligned Polymer Nanofiber-Wrapped Glass Microfibers for Cortical Bone Tissue Engineering. Bioengineering 2020; 7(4): 165
Open Access
-
Liu J, Zou Q, Wang C, Lin M, Li Y, Zhang R, Li Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Materials & Design 2021; 210: 110047
Open Access
-
Liu Y, Zhang B, Liu F, Qiu Y, Mu W, Chen L, Ma C, Ye T, Wang Y. Strontium doped electrospinning fiber membrane with antibacterial and osteogenic properties prepared by pulse electrochemical method. Engineered Regeneration 2022; 3: 339.
Open Access
-
Mellor L F, Nordberg R C, Huebner P, Mohiti-Asli M, Taylor M A, Efird W, Oxford J T, Spang J T, Shirwaiker R A, Loboa E G. Investigation of multiphasic 3D-bioplotted scaffolds for site-specific chondrogenic and osteogenic differentiation of human adipose-derived stem cells for osteochondral tissue engineering applications. J Biomed Mater Res. 2020;108B:2017.
Open Access
-
Ngiam M L M. Differentiation of Bone Marrow Derived Mesenchymal Stem Cells (BM-MSCs) using Engineered Nanofiber Substrates. PhD Thesis, National University of Singapore 2010.
Open Access
-
Pensa N W, Curry A S, Bonvallet P P, Bellies N F, Rettig K M, Reddy M S, Eberhardt A W, Bellies S L. 3D printed mesh reinforcements enhance the mechanical properties of electrospun scaffolds. Biomater Res 2019; 23: 22.
Open Access
-
Ravichandran R. Biomimetic Surface Modification of Dental Implant for enhanced Osseointegration. MEng Thesis. National University of Singapore 2009.
Open Access
-
Soujanya G K, Hanas T, Chakrapani V Y, Sunil B R, Kumar T S S. Electrospun Nanofibrous Polymer Coated Magnesium Alloy for Biodegradable Implant Applications. Procedia Materials Science 2014; 5: 817.
Open Access
-
Tao F, Cheng Y, Tao H, Jin L, Wan Z, Dai F, Xiang W, Deng H. Carboxymethyl chitosan/sodium alginate-based micron-fibers fabricated by emulsion electrospinning for periosteal tissue engineering. Materials & Design 2020; 194: 108849.
Open Access
-
Teo W E, Gopal R, Ramaseshan R, Fujihara K, Ramakrishna S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer 2007; 48: 3400.
-
Teo W E, Liao S, Chan C, Ramakrishna S. Fabrication and characterization of hierarchically organized nanoparticle-reinforced nanofibrous composite scaffolds. Acta. Biomater. 2011; 7: 193.
▲ Close list
 ElectrospinTech
ElectrospinTech

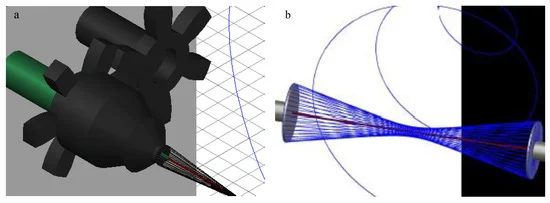 AutoCAD images of (a) the final design of the modified air-gap electrospinning with motor setup, signifying the stationary (green) and rotating (black) parts. (b) A simplified setup demonstrating the wrapping of electrospun fibers (blue) around the glass fiber (red) [Linder et al 2020]
AutoCAD images of (a) the final design of the modified air-gap electrospinning with motor setup, signifying the stationary (green) and rotating (black) parts. (b) A simplified setup demonstrating the wrapping of electrospun fibers (blue) around the glass fiber (red) [Linder et al 2020]