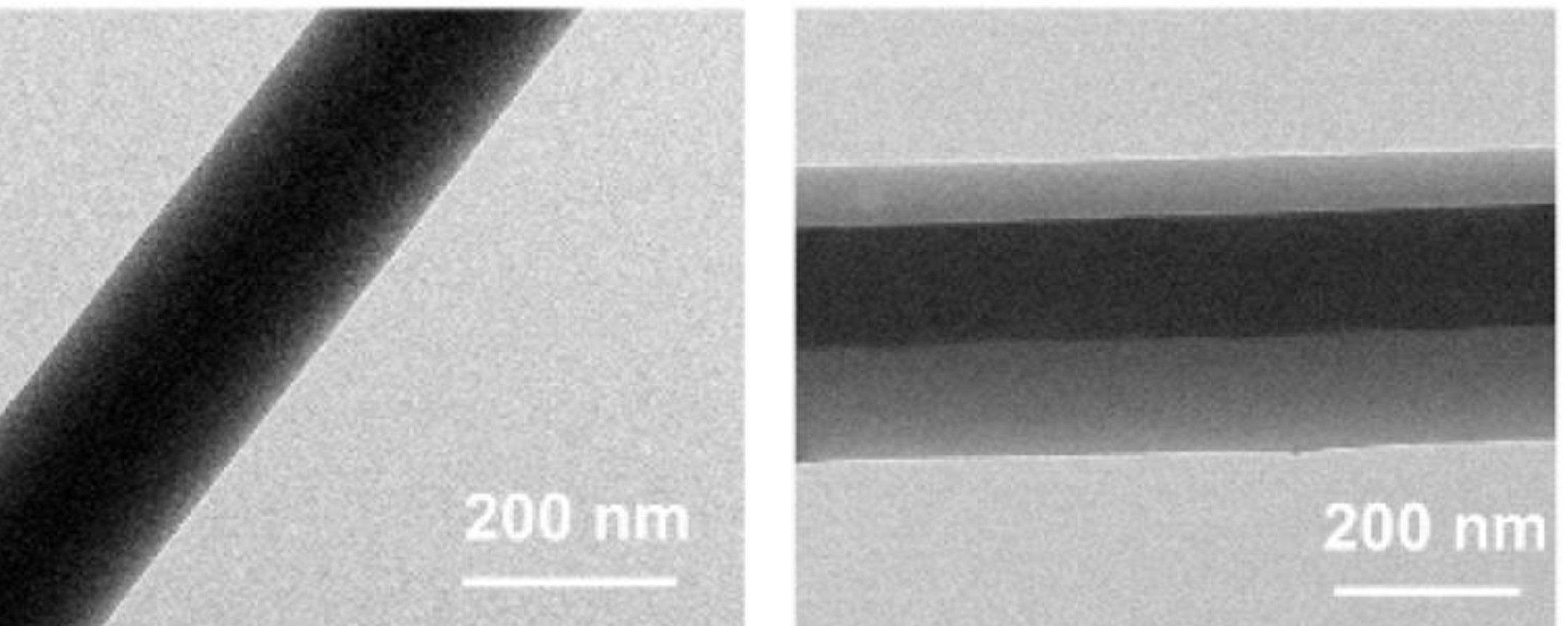
Electrospinning is commonly used for encapsulating drugs for delivery. There are numerous studies on the release rate of the drugs in different polymers and while some show initial burst release of the drug, others showed a more controlled release of the drug over a longer duration. For sustained drug release, the release kinetic of the drug will give an indication of the drug release profile across the duration. In many cases, a zero-order kinetic with constant release rate is preferred. Drug release profile in electrospun fiber matrix is dependent on many factors. The same drug but loaded into different matrix materials may exhibit different release profile. Mirzaeei et al (2018) electrospun fibrous meshes of chitosan/polyvinyl pyrrolidone (PVP), chitosan/PVP/polyvinyl alcohol (PVA) and zein/Eudragit loaded with triamcinolone acetonide for the purpose of ocular delivery. Of these, chitosan/PVP electrospun fibers showed a sustained release up to 4 days while zein/Eudragit fibers released 77% of the drugs within this period. Investigation into the release kinetics of electrospun fibrous meshes of triamcinolone acetonide loaded chitosan/polyvinyl pyrrolidone (PVP) electrospun nanofibers showed the preferred Zero-order kinetic with constant release rate and independent drug concentration while other polymer combinations adhere to Higuchi model. There are many factors that influence the drug release profile of the carrier and this will be introduced in this article.
Degradation/dissolution rate
The degradation and dissolution rate of the polymer matrix has a significant influence on the drug release rate. Water soluble polymers loaded with drugs and spun into fibers have been used as a quick release carrier for oral drug delivery [Dott et al 2013]. Ball et al (2012) used a combination of hydrophilic polyethylene oxide (PEO) and hydrophobic poly-L-lactic acid (PLLA) to control the release rate of loaded drugs. Release of the drugs within an aqueous media showed that the release rate is slower with higher PLLA content in the fiber matrix. Pure PLLA fibers showed no release of maraviroc drug before 96 hours [Ball et al 2012].
The use of a water soluble component may also facilitate the complete release of active ingredients instead of entrapping and slow release of clinically insignificant dosage at the tail end of the release profile. Yan et al (2014) used a mixture of cellulose acetate and water soluble polyvinyl pyrrolidone (PVP) to control the release rate of ferulic acid (FA), a poorly water-soluble active ingredient. With increased ratio of PVP, the release rate of FA increases. The presence of water soluble PVP in the fiber matrix creates pores within the fibers which expose the FA to the surrounding media for sustained and complete release. Without the inclusion of PVP, it takes more than 70 hrs for the last 10% of the drug to be released while it takes less than 35 hrs where PVP makes up 8% of the matrix.
Annealing and increasing crystallinity of polymer matrix would reduce the degradation rate and solubility. Li et al (2017) was able to reduce the release rate of curcumin (CUR) in electrospun regenerated silk fibroin (RSF) by water annealing to reduce the solubility of electrospun RSF in release medium which comprised of phosphate buffer saline (PBS) and ethanol. Comparing the release rate of CUR between fibers annealed at 45 ° and 60 °, the former release rate was relatively faster than the latter. The difference in release rate was attributed to lower crystal content at lower temperature annealing which allows more water molecules to penetrate into the RSF matrix.
There are several types of biodegradable polymers with varying rate of degradation. Polyglycolic acid (PGA) will degrade completely within a few days, polylactic acid (PLA) a few months and polycaprolactone (PCL) a few years. These and their copolymers and other types of polymers give a diverse range of degradation and corresponding drug release rates. To tailor the drug release rate of anti-glioma drug Temozolomide (TMZ), Ramachandran et al (2017) used a mixture of copolymer poly(lactic-glycolic acid) (PLGA) with different lactic to glycolic ratio, polylactic acid (PLA) and PCL to control the electrospun fiber degradation rate. With higher glycolic content in the PLGA copolymer, the burst release of the drugs are greater, probably due to faster degradation of PGA. Increasing slower degrading lactic acid content reduces the burst release. Addition of small amount of PLA and PCL into the electrospun fiber blend was able to control burst release.
Another way of controlling the degradation/dissolution rate of the matrix material is by chemical cross-linking. Chemically cross-linked materials have reduced sites available for reaction with the environment which makes it more stable. Nada et al (2016) used a blend of hydroxyethyl cellulose (HEC) and polyvinyl alcohol (PVA) for loading of topical drug, nicrotinamide. Both HEC and PVA are water soluble and fast release of the drug can be expected without any further chemical modification. Without any chemical cross-linking, 80% of the drug may be released after 2 hours. However, when citric acid was added for cross-linking, the resultant scaffold took 24 hours to release more than 80% of the drug.
Certain chemical cross-linking is effective in increasing hydrophobicity and reducing degradation rate of electrospun membranes which in turn reduces its drug release rate. Han et al (2019) cross-linked lutein-loaded polyvinyl alcohol/sodium alginate (PVA/SA) nanofibers using a mixture of glutaraldehyde and saturated boric acid solution at room temperature to alter its drug release rate. With longer cross-linking duration, the longer the sustained drug release. The drug release mechanism may also be altered by the cross-linking duration. The drug release rate from a cross-linked duration of 1h and 5h was 12.5% per hour and 0.85% per hour respectively. For a cross-linking duration of an hour, the PVA/SA nanofibers remain hydrophilic and drug release is determined by diffusion and dissolution of the polymer in water. When the cross-linking duration is 5h, the nanofibrous membrane becomes hydrophobic and the main release mechanism is from diffusion.
Drug release rate may also be controlled by the degree of swelling of the matrix material in water. When hydrophilic material absorbs water and swells, it opens up the pores and facilitates diffusion of loaded drugs within the matrix. Ko et al (2019) electrospun blend of polyurethane (PU) and cellulose acetate (CA) with paclitaxel (PTX) as the model drug. PU has good mechanical properties while CA is hydrophilic with high water uptake. With higher content of CA in the electrospun PU/CA composite, swelling of the electrospun membrane increases. Increased swelling also led to a corresponding increase in drug release. Comparing the drug release profile of electrospun PU, PU/CA (7:3) and PU/CA (3:7) membrane, they shared similar initial burst release rate before diverging. The similarity in the drug release rate at the initial phase can be attributed to drugs that are at the surface of the nanofibers. Once these exposed drugs were released, subsequent release is dependent on the diffusion of the drugs across the matrix.
Drug/polymer matrix compatibility
The nature of the drug and its compatibility with the matrix has a strong influence on the release rate. In drug release from a partially water-soluble polymer such as polyvinyl alcohol (PVA), a hydrophobic drug may be released at a much slower rate than a more hydrophilic drug. Blakney showed that the in vitro release of the more hydrophilic tenofovir (TFV) is completed within 30 minutes but the complete release of hydrophobic levonorgestrel (LNG) took 4 hours.
Thao et al (2021) loaded dexamethasone (Dex) and silver sulfadiazine (AgS) into electrospun small intestinal submucosa (SIS)/poly(ε-caprolactone-ran-l-lactide) (PCLA) (5:1) membrane. In an in vivo drug release test, Dex, which is more water soluble than AgS, saw a rapid release on the first 3 days (40%) followed by linear release of the remaining amount over 14 days. For AgS, 37% was released on the first 3 days with little release over 21 days. The more water soluble Dex may have greater affinity towards SIS in the polymer mixture which has a higher degradation rate. Since AgS is poorly water soluble, more of it may get trapped in PCLA and the slow degradation of PCLA would have slowed its release after the initial burst release. Degradation test using collagenase showed the SIS sheet completely degraded after 1 day, electrospun SIS/PCLA (5:1) membrane showed 50% degradation in a day but remained the same for 3 days and electrospun PCLA membrane showed no degradation. For a membrane made of SIS and PCLA, the initial fast degradation is likely due to the degradation of SIS and the degradation rate slows down significantly due to slow degradation of the remaining PCLA.
Drug distribution and barriers
In general, drugs distribution within the fibers may be near the surface, at the core or uniformly distributed throughout the matrix. Blending of drugs in the polymer solution for electrospinning is the most commonly used technique of loading fibers with drug. This generally results in uniform distribution of the drugs across the fiber matrix. Interaction between the polymer and the drugs within the solution may influence the distribution of the drugs. Sonication prior to electrospinning may be needed to improve uniform distribution of the drugs. When drug distribution is more uniform in the electrospun fiber matrix, a more gradual release rate may be expected. Gohary et al (2018) demonstrated this effect with doxorubicin (dox) loaded silica/poly(ε-caprolactone)/polyethylene oxide hybrid fiber mats. As loaded silica nanoparticles in the solution for electrospinning without sonication showed agglomeration on the resultant electrospun fibers. The release of dox in this fiber mat was about 69% at day 33 compared to 58% from fibers electrospun from sonicated solution. In core-shell electrospun fibers, the drugs may be deliberately localized either at the core or at the shell of the fibers. Drugs confined to the core of the fiber will take a longer time to release as there is a thicker barrier for them to pass through.
Ding et al (2020) constructed an electrospun core-shell fiber with both the core and shell made of ES100 but the drug, aspirin, is only loaded into the core of the fiber. The presence of the outer ES100 wall provides an additional barrier for the inner aspirin to cross over before it is being released. When compared with homogeneously distributed aspirin in ES100 electrospun fibers, the core-shell fibers release a lower dose of aspirin in the first 2 hours against the former. There is a longer sustained release of aspirin for core-shell fibers with 50% and 95% drug release at 4.15 and 6.09 h respectively. This compared well to uniformly distributed drugs in the fiber with 50% and 95% drug release at 2.66 and 3.91 h respectively.
The type of shell material used to form the core-shell would also influence the rate of drug release from the core. Mares-Bou et al (2023) used poly (L-lactic acid) (PLLA) and polycaprolactone (PCL) as shell materials with polyvinyl alcohol (PVA)/bovine serum albumin (BSA) core for the release of BSA. PLLA has a faster degradation rate compared to PCL hence PLLA as the shell showed a faster BSA release rate. The fastest release rate was from PVA/BSA fibers without any shell coating. Therefore the release rate can be regulated by the presence and type of shell coating over a drug loaded core matrix.
Liu et al (2022) electrospun core-shell cellulose acetate (CA) with CA loaded with curcumin at the core. Instead of using a coaxial nozzle, a triaxial nozzle was used and the outermost layer was a solvent mixture of ethanol, DMF, and acetone. The outermost coverage by the solvents help to maintain the stability of the electrospinning process. The flow rate of the middle section containing pure CA was varied to control the thickness of the wall covering the innermost CA loaded fibers. The electrospun fibers from blended curcumin in CA, showed an initial burst release. The introduction of a layer covering the drug loaded core prevented initial burst release and a thicker sheath layer showed an almost linear release from 12 to 36 h.
In a core-shell setup, having the core and shell made of different materials may help to prevent minute amounts of molecules encapsulated at the core from leaching out. Abdelhakim et al (2021) used co-axial electrospinning to create a core-shell fiber for the purpose of taste masking a bitter drug. Presence of bitterness would indicate that a minute amount of the drug has leached to the surface. It was found that having Kollicoat® Smartseal (KCT) in the core with the drug and Eudragit® E PO (E-EPO) in the shell is the most effective for taste masking. E-EPO electrospins well hence it is able to form an intact and uniform covering over the KCT/chlorpheniramine maleate core and provide effective taste-masking. When E-EPO was used as both the core and the shell, the masking effect was less than the use of two different polymers. When the core and shell are made of different materials, there will be an interface between those two which retards the migration of the drug to the surface.
Encapsulation of drugs within micelles or nanoparticles prior to blending into electrospun solution may also be used to introduce an additional barrier to drug migration to the fiber surface [Yang et al 2014]. Castro-Dominguez et al (2017) used the concept of mixed matrix membranes (MMMs) to control release of active pharmaceutical ingredients (API). This typically involves having a polymeric membrane with fillers dispersed into its matrix to alter the API release rate. Castro-Dominguez et al (2017) electrospun poly ε-caprolactone (PCL) as the membrane containing zeolite as the molecular sieve fillers and ibuprofen as the API. Comparing the release characteristic of ibuprofen in electrospun PCL containing zeolite and without, the membrane containing zeolite showed a much slower release rate. This is probably due to the stronger van der Waal attraction between ibuprofen and zeolite.
On the other hand, if it is desirable to have a faster release rate when the release of the drug is slow, steps may be taken to increase the amount of surface exposed drug. Song et al (2012) introduced silica nanoparticles into the electrospun fibers loaded with drugs. The presence of the silica nanoparticles creates bumps when they are near the surface of the fibers. This has the effect of pushing more drugs to the surface of the fibers and a greater initial drug release was observed.
The thickness of the shell of the core-shell fibers can be tailored by varying its flow rate. By increasing the shell thickness, the release rate of the active ingredients from the core can be reduced. Zhou et al (2017) investigated the release rate of Si from silicon doped vaterite (SiV) dispersed in poly(L-lactic acid) as the core and poly(D,L-lactide-co-glycolide) (75:25) (PLGA) as the shell material. The release rate of ions from varying thickness of the shell material was found to follow the Weibull model which suggests the mechanism off release is purely diffusive. Therefore, a thicker PLGA shell was able to significantly reduce the ion release rate. Where the shell thickness was 1.3 and 2.3 µm, 85% and 60% of the Si ions were released in the first two days respectively. Without the shell almost all the Si ions were released within a day. The release rate may also be controlled by the type of shell material. Pedersbaek et al (2017) showed that for the drug, tetracycline hydrochloride (TCH), its release rate depends on whether the shell material is poly-lactic acid (PLA) or poly-ε-caprolactone (PCL). When the shell material was PCL, all the TCH in the core was released quickly. However, when the shell was added with 10 wt% PLA to PCL, the release of TCH was significantly retarded. By varying the ratio between PLA and PCL in the shell material, the amount of TCH burst release can be controlled. TCH was able to diffuse through PCL quickly but not in PLA. However, the reason behind this is unclear.
In core-shell fibers, it is more common to have the drug loaded in the core and the shell be used to control the release rate of the drug. Having the drug loaded in the shell instead will have a different release profile compared to uniaxial fibers. Nagiah et al 2020 showed that the release of model small molecule rhodamine B (RhB) released from the shell layer was faster than the release rate from uniaxial fiber. The cumulative release of RhB from the shell layer of core-shell fiber (50%) was also greater than the cumulative release from uniaxial fiber (20%). The higher release of RhB may be attributed to thinning and spreading of the RhB loaded polymer matrix over the inner core fiber material of a core-shell fiber. Since the thickness of the shell layer in the core-shell fiber is smaller than the radius of a uniaxial fiber, the distance which the RhB molecules are required to travel from the interior to the surface is much smaller.
A fiber with a concentric layer also known as core-shell fiber has shown the ability to control drug release by selecting the location where the drug is loaded and the type of polymer material found in the core or the outer layer. Having more concentric layers will introduce more complexity and opportunities to control the drug release profile. Introducing more concentric layers to the fiber will generally increase the fiber diameter [Nagiah et al 2020] but this may be compensated by the greater variation in drug release characteristic. Nagiah et al 2020 investigated the drug release characteristic of triaxial electrospun fiber membrane for model small molecule rhodamine B (RhB) released from the sheath layer and a large molecule fluorescein isothiocynate (FITC) bovine serum albumin (BSA) conjugate in the intermediate layer. The triaxial fiber will have polycaprolactone (PCL) (core fiber layer), 50:50 poly(lactic-co-glycolic acid) (PLGA) (sheath layer) and gelatin (intermediate layer). Although RHB is a smaller molecule, it is less hydrophilic compared to BSA-FITC. A fiber layer containing BSA-FITC will have a higher water retention and this increases its release rate. Having hydrophilic RHB in the sheath has been shown to increase the release rate of BSA-FITC in the core and inner layer. It is also possible that the release of RHB molecules from the sheath may have also created voids for faster passage of BSA-FITC through the sheath layer. BSA-FITC cumulative release in the presence of RhB in the sheath layer was more than 60% while in the absence of RhB, the cumulative release of BSA-FITC was just 40%.
Instead of controlling the drug release rate within a single fiber, another method is to use a mixture of fibers or different fiber layers containing the drugs. Umi-i-Zahra (2015) created a penta-layered nanofiber meshes with layers comprising of cellulose acetate (CA), polyvinyl pyrrolidone (PVP) and ethyl cellulose fibers (EC). By sandwiching a layer of drug loaded PVP between layers of CA and EC, a spike in the drug released has been demonstrated in the middle of the drug release period when the layered fibrous structure was immersed in PBS buffer solution.
To reduce the drug release rate in a electrospun nanofiber drug loaded layer, the covering layers may be made of more hydrophobic material. Habibi et al (2018) demonstrated this effect with a three layered drug-loaded biodegradable nanofibrous scaffolds comprising of polylactic acid (PLA) (top)/ drug- loaded polyvinyl alcohol (PVA) (second) and PLA-PVA (bottom) nanofibers for sustained release of naltrexone. Since PLA is hydrophobic, a thicker layer of electrospun PLA or a greater concentration of PLA in the mixture result in slower naltrexone release rate. Conversely, adding more PVA which is water soluble to the mixture increase the drug release rate due to greater water penetration. At optimal parameters, sustained release of the drug lasts more than 30 days. The release mechanism of naltrexone in this model was through Fickian diffusion.
The thickness of electrospun membrane containing the drug have also been shown to affect the drug release profile. Immich et al (2017) showed that with electrospun PLLA loaded with caffeine, there is an initial burst release followed by a stable release. This initial burst release was found to be independent of amount of caffeine loaded. With thicker electrospun PLLA fiber membrane, the time to reach stable release rate was longer at 200 minutes for thicker PLLA membrane, between 0.1192 and 0.1655 mm compared to immediate complete release for membrane thickness between 0.0662 and 0.1190 mm. With greater membrane thickness, the interstitial space required for the water to penetrate to the core and for the drug to navigate to the surface increases.
Javazmi et al (2021) showed that a sandwich assembly of electrospun membranes is very effective in reducing the release rate of urea fertiliser. In their assembly, an electrospun single-layer poly L-lactic acid (PLLA) nanofibres membrane loaded with urea was sandwiched between two electrospun layers of polyhydroxybutyrate (PHB) nanofibres membranes. With single layer PLLA/Urea, more than 50% of the urea was released within 3 hours. For sandwiched PLLA membrane loaded with 10% urea, less than 50% was released after 39 hours. However, with higher urea loading (20% and 40%), the sandwiched PLLA membrane release rate has a much faster release rate comparable to that of single layer PLLA membrane. Higher urea loading resulted in a much faster release rate. In a sandwiched assembly, the PHB layers provide a barrier that slows the diffusion of urea. Hydrophobicity of electrospun PHB and PLLA at lower urea loading may reduce the rate of water penetration which in turn reduces the rate of urea release. Since urea is water soluble, higher concentration of urea in PLLA may reduce the hydrophobicity of the membrane. In a sandwich assembly, once water has penetrated the PHB layer, the PLLA/urea membrane may have facilitated wetting and hence increase its urea release.
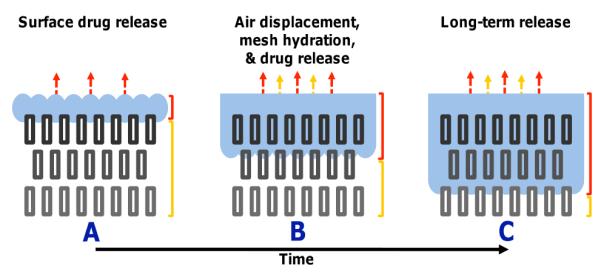
The hydrophobicity of a membrane will have an influence on the rate of water penetration into its depth. In the Cassie-Baxter model, hydrophobicity of a material is due to air pockets between the pores of a material. In electrospun membrane, the pores formed by the interconnecting fibers were able to trap air and this makes membrane made of non-water absorbent material hydrophobic. As diffusion of embedded substances requires contact with water or fluid, increasing the time taken for water penetration into the membrane will certainly reduces the rate of drug release. Yohe et al (2012) electrospun poly(ε-caprolactone) (PCL)/poly(glycerol monostearate-co-ε-caprolactone) ( PGC-C18) to investigate the effect of hydrophobicity in the release of a model bioactive agent (SN-38). Comparing the release rate of SN-39 for 10% doped PGC-C18 electrospun meshes, melted 10% doped PGC-C18 electrospun meshes and degassed 10% doped PGC-C18 electrospun meshes, the time taken for the release of 50% of SN-38 was about 40 days, 12 days and 1 day respectively. This shows that the pores within the electrospun mesh played an important role to spread the drug release over a longer duration.
Response to environmental conditions
Electrospun fiber material may be selected to respond to different environmental conditions such as pH and temperature. Biodegradable pH-sensitive polymers containing ortho ester groups such as D,L-lactide have been shown to increase the drug release rate in acidic media [Qi et al 2008]. Yuan et al (2014) constructed a pH responsive polyL-lactide nanofiber containing sodium bicarbonate (NaHCo3) for faster release of ibuprofen. EUDRAGIT® are synthetic polymers containing ratio ranging from two to three methacrylate monomers, such as methacrylic acid, methacrylic acid esters, and dimethylaminoethyl methacrylate with customized solubility in different pH environments. EUDRAGIT® polymers may be electrospun into fibers with drug loaded for release at targeted organ based on their pH. Further mixing of different EUDRAGIT® polymers may be used to alter the rate and amount of drug release. Vlachou et al (2019) used various EUDRAGIT® and their mixtures for the release of furosemide, a chloride channel blocker generally used as a high-ceiling or loop diuretic. Electrospun EUDRAGIT® containing furosemide showed that furosemide is amorphous in the fiber. Of the various forms of EUDRAGIT® used, the relative percentage of E100 (poly(butyl methacrylate-co-(2-demethylaminoethyl) methacrylate-co-methyl methacrylate) 1:2:1) in the electrospun EUDRAGIT® polymer mixture modulated the percentage release of furosemide both at pH 6.8 and 1.2. EUDRAGIT® E100 is acidic pH-dependent. At low pH of 1.2, the presence of E100 increases furosemide release. Conversely, at higher pH of 6.8, higher amount of E100 reduces furosemide release. Sayin et al (2019) used a coating on electrospun fibers to control drug release according to pH. Using water-soluble polyvinyl alcohol (PVA) as the carrier material for chemotherapeutic agent Rose Bengal (RB) (4,5,6,7-tetrachloro-2', 4',5',7'-tetraiodofluoresceindisodium), a thin layer of poly(4-vinylpyridine-co-ethylene glycol dimethacrylate) p(4VP-co-EGDMA) was deposited on its surface to control its solubility. In PBS solution, uncoated PVA nanofibers released 80% of loaded RB in 1 h regardless of pH. In the presence of p(4VP-co-EGDMA) coating, RB has to travel through the PVA matrix and the coating. The coating p(4VP-co-EGDMA) is sensitive to pH with greater stability at pH 6.5 and above. At lower pH, the p(4VP-co-EGDMA) swells. This behavior of p(4VP-co-EGDMA) has a direct influence in the release of RB. At high pH, the release of RB is faster as the coating is at its thinnest as given by 98% of the RB released at the end of 6 h at pH 9. At low pH, p(4VP-co-EGDMA) swells and this increases the distance which RB needs to travel hence reducing its release rate. This resulted in only 55% of RB released in 6 h at pH 4. Another reason for such low release rate at low pH may be attributed to protonation of pyridine groups of p(4VP) which led to electrostatic interaction between the protonated pyridine groups and the RB molecules.
Thermoplastic polymer which exhibit changes at temperature close to human body temperature of 37°C is particularly suited for thermos-responsive drug release. Azarbayjani et al (2010) used a mixture of polyvinyl alcohol and poly(N-isopropylacrylamide) (PNIPAM) to control the release of Levothyroxine [3, 5, 3', 5'-tetraiodothyronine], is a synthetic hormone used for the treatment of hypothyroidism and goiter. PNIPAM is a thermally reversible hydrogel with a lower critical solution temperature of 32°C. This material swells below this temperature and shrinks above it. When the material shrinks, drug release is expected to increase.
Response to artificial trigger
Electrospun fibers loaded with drugs may also be loaded with other materials that can response to external triggers. Song et al (2015) embedded silica nanoparticles into drug loaded electrospun fibers ultrasound triggered drug release. In their experiment they showed that with the application of ultrasound, drug release rate increases. This has been attributed to heating of the buffer solution which increases the release kinetics of the drugs. Electrospun polymer may be incorporated with light sensitive additives to initiate changes in response to light. Ramanan et al (2011) constructed a nanofibrous composite of poly(N-isopropylacrylamide-co-polyethylene glycol acrylate) (PNPA) and PEGylated gold nanorods (AuNRs) which significantly increases the release of incorporated proteins when triggered under near infrared red light. AuNRs was able to strongly absorb near infrared red light to generate heat and this triggered a thermal transition in the polymer matrix. With the heat, the PNPA fibers shrink and the resultant expulsion of water causes an increased release of protein that is tenfold that of the composite fibers without stimulus.
Drug combinations
The ease of loading drugs into electrospun fibers have encouraged researchers to test combination drug loading into the fibers. The release rate of the drugs when used in combination may differ from when they are loaded separately in the fibers. Blakney et al (2014) found that when tenofovir (TFV) and levonorgestrel (LNG) were blended together and electrospun in polyvinyl alcohol (PVA) solution, the release rate of TFV was slower than when TFV alone was loaded into the PVA fiber matrix. This has been attributed to the more hydrophobic nature of LNG in the matrix which reduces the release of TFV. Li et al (2017) showed that having doxorubicin hydrochloride (DOX·HCl, hydrophilic drug, in the shell of a core-shell fibers would reduce the release rate of curcumin (CUR, hydrophobic drug) in CUR-loaded regenerated silk fibroin (RSF) nanosphere embedded in the core of electrospun RSF fibers. Li et al (2017) suggested that a higher drug density due to the presence of DOX-HCI reduces water molecule permeability into the fiber and this impeded the release rate of CUR.
Electrospinning flow rate
There are several processing parameters that affect the electrospinning outcome. In solution rate, typically a higher solution flow rate will bring about larger fiber diameter. With a larger fiber diameter, one would expect the release rate would be slower due to the lower surface area to volume. However, this may not always be the case. Ranjith et al (2017) showed that with naringin loaded electrospun polycaprolactone nanofibers, a higher flow rate and with the corresponding increase in fiber diameter, the cumulative release of naringin was greater on 12 day. Although the author did not explain why this is so, this may be due to greater amount of naringin at the surface of the fibers which gives a larger initial burst release for the larger diameter fiber.
Fiber assembly
In drug release, one of the most important factors influencing the release profile is the surface area. In general, the larger the surface area, the quicker the release rate. While reduction in the electrospun fiber diameter will increase the surface area, the fiber assembly will also have an effect on the surface area on the macro level.
As a flat membrane, the surface texture or roughness can be easily changed by the collecting substrate. Grids and protrusion on the substrate will be translated on the electrospun membrane. Saadatmand et al (2019) tested the effect of textured electrospun membrane on the release rate of cetirizine in Nylon 6 fibers. Electrospun Nylon 6 fibers were deposited on smooth surface substrate, pentagonal grids and tetragonal grids. From their study, they found that drug release from membrane with pentagonal profiling has the fastest release rate and followed a first order release model where the release rate is concentration dependent. Both smooth surface and tetragonal profile membrane followed a Higuchi model where the release rate is slower. Checking the surface roughness value showed that the pentogonal profile membrane (4.16 µm) has the highest value compared to smooth surface electrospun membrane (0.814 µm) and tetragonal surface profile electrospun fibers (2.29 µm). Therefore, the surface profile and its effect on roughness influences the drug release rate of electrospun membranes.
Published date: 19 July 2016
Last updated: 30 January 2024
▼ Reference
-
Abdelhakim HE, Coupe A, Tuleu C, Edirisinghe M, Craig DQM. Utilising Co-Axial Electrospinning as a Taste-Masking Technology for Paediatric Drug Delivery. Pharmaceutics. 2021; 13(10):1665.
Open Access
-
Azarbayjani A F, Venugopal J R, Ramakrishna S, Lim P F C, Chan Y W, Chan S Y. Smart Polymeric Nanofibers for Topical Delivery of Levothyroxine. J Pharm Pharmaceut Sci 2010; 13: 400.
Open Access
-
Ball C, Krogstad E, Chaowanachan T, Woodrow K A. Drug-Eluting Fibers for HIV-1 Inhibition and Contraception. PLoS ONE 2012; 7(11): e49792.
Open Access
-
Blakney A K, Krogstad E A, Jiang Y H, Woodrow K A. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. International Journal of Nanomedicine 2014; 9: 2967. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4069153/
-
Castro-Dominguez B, Moroney K, Schaller B, O'Connor S, Cloonan A, Vo T T N, Walker G, O'Reilly E J. Electrospun API-loaded mixed matrix membranes for controlled release.
RSC Adv. 2017; 7: 43300.
Open Access
-
Ding Y, Dou C, Chang S, Xie Z, Yu DG, Liu Y, Shao J. Core-Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020; 12: 2034.
Open Access
-
Dott C, Tyagi C, Tomar L K, Choonara Y E, Kumar P, Toit L C, Pillay V. A Mucoadhesive Electrospun Nanofibrous Matrix for Rapid Oramucosal Drug Delivery. Journal of Nanomaterials 2013; 2013: 924947.
Open Access
-
Gohary M I E, Hady B M A E, Saeed A A A, Tolba E, Rashedi A M I E, Saleh S. Electrospinning of doxorubicin loaded silica/poly(ε-caprolactone) hybrid fiber mats for sustained drug release. Advances in Natural Sciences: Nanoscience and Nanotechnology 2018; 9 (2).
Open Access
-
Habibi M, Irani M, Samira Jafari S, Haririan I. Controlled release of Naltrexone using three layered drug-loaded PVA/PLA nanofibrous scaffolds. Journal of Advanced Chemical and Pharmaceutical Materials. July 25, 2018
Open Access
-
Han X, Huo P, Ding Z, Kumar P, Liu B. Preparation of Lutein-Loaded PVA/Sodium Alginate Nanofibers and Investigation of Its Release Behavior. Pharmaceutics 2019; 11: 449.
Open Access
-
Immich A P S, Tornero J A, Casas F C, Arias M J L. Electrospun PLLA Membranes for Caffeine Delivery: Diffusional Approach. JBISE 2017; 10: 563
Open Access
-
Javazmi L, Young A, Ash G J, Low T. Kinetics of slow release of nitrogen fertiliser from multi-layered nanofibrous structures. Sci Rep 2021; 11: 4871.
Open Access
-
Ko SW, Lee JY, Lee J, Son BC, Jang SR, Aguilar LE, Oh YM, Park CH, Kim CS. Analysis of Drug Release Behavior Utilizing the Swelling Characteristics of Cellulosic Nanofibers. Polymers 2019; 11: 1376.
Open Access
-
Li H, Jingxin Zhu, Chen S, Jia J, Ma Y. Fabrication of aqueous-based dual drug loaded silk fibroin electrospun nanofibers embedded with curcumin-loaded RSF nanospheres for drugs controlled release. RSC Adv. 2017; 7: 56550.
Open Access
-
Liu Y, Chen X, Gao Y, Liu Y, Yu D, Liu P. Electrospun Core-Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules. 2022 Jul 29;12(8):1057.
Open Access
-
Mares-Bou S, Serrano M-A, Gómez-Tejedor JA. Core-Shell Polyvinyl Alcohol (PVA) Base Electrospinning Microfibers for Drug Delivery. Polymers. 2023; 15(6):1554.
Open Access
-
Mirzaeei S, Berenjian K, Khazaei R. Preparation of the Potential Ocular Inserts by Electrospinning Method to Achieve the Prolong Release Profile of Triamcinolone Acetonide. Adv Pharm Bull. 2018 Mar; 8(1): 21.
Open Access
-
Nada A A, Hassabo A G, Mohamed A L, Zaghloul S. Encapsulation of Nicotinamide into Cellulose Based Electrospun Fibers. J App Pharm Sci. 2016; 6: 013.
Open Access
-
Nagiah N, Murdock C J, Bhattacharjee M, Nair L, Laurencin C T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Scientific Reports 2020; 10: 609.
Open Access
-
Pedersbaek D, Frantzen M T, Fojan P. Electrospinning of Core-Shell Fibers for Drug Release Systems. Journal of Self-Assembly and Molecular Electronics (SAME) 2017; 5: 17.
Open Access
-
Qi M, Li X, Yang Y, Zhou S. Electrospun fibers of acid-labile biodegradable polymers containing ortho ester groups for controlled release of paracetamol. European Journal of Pharmeutics and Biopharmaceutics 2008; 70: 445.
-
Ramachandran R, Junnuthula V R, G. Gowd S, Ashokan A, Thomas J, Peethambaran R, Thomas A, Unni A K K, Panikar D, Nair S V, Koyakutty M. Theranostic 3-Dimensional nano brain-implant for prolonged and localized treatment of recurrent glioma. Scientific Reports 2017; 7: 43271.
Open Access
-
Ramanan V V, Hribar K C, Katz J S, Burdick J A. Nanofiber-nanorod composites exhibiting light-induced reversible lower critical solution temperature transitions. Nanotechnology 2011; 22: 494009.
-
Ranjith R, Balraj S, Ganesh J, Milton M C J. Effect of Flow Rate on Fiber Morphology and Naringin Release of Electrospun Naringin Loaded Polycaprolactone Nanofibers. IJSRST 2017; 3: 2395.
Open Access
-
Saadatmand M M, Yazdanshenas M E, Khajav R, Mighani F, Toliyat T. Patterning the surface roughness of a nano fibrous scaffold for transdermal drug release. International Journal of Nano Dimension 2019: 10: 78.
Open Access
-
Sayin S, Tufani A, Emanet M, Genchi G G, Sen O, Shemshad S, Ozdemir E, Ciofani G, Ince G O. Electrospun Nanofibers With pH-Responsive Coatings for Control of Release Kinetics. Front Bioeng Biotechnol. 2019; 7: 309.
Open Access
-
Song B, Wu C, Chang J. Dual drug release from electrospun poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles composite mats with distinct release profiles. Acta Biomaterialia 2012; 8: 1901.
-
Song B, Wu C, Chang J. Ultrasound-triggered dual-drug release from poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles electrospun composite fibers. Regenerative Biomaterials 2015; 1-9.
-
Thao NTT, Lee S, Shin GR, Kang Y, Choi S, Kim MS. Preparation of Electrospun Small Intestinal Submucosa/Poly(caprolactone-co-Lactide-co-glycolide) Nanofiber Sheet as a Potential Drug Carrier. Pharmaceutics. 2021;13(2):253.
Open Access
-
Um-i-Zahra S. Study of in-Vitro Drug Release Profiles of Time-controlled Penta-layered Nanofiber Meshes. International Journal of Research 2015; 2: 152.
Open Access
-
Yan J, White K, Yu D G, Zhao X Y. Sustained-release multiple-component cellulose acetate nanofibers fabricated using a modified coaxial electrospinning process. J. Mater. Sci. 2014; 49: 538.
-
Yang G, Wang J, Li L, Ding S, Zhou S. Electrospun Micelles/Drug-Loaded Nanofibers for Time-Programmed Multi-Agent Release. Macromol. Biosci. 2014; 14: 965.
-
Yohe S T, Colson Y L, Grinstaff M W. Superhydrophobic Materials for Tunable Drug Release: Using Displacement of Air to Control Delivery Rates. J Am Chem Soc. 2012; 134(4): 10.
Open Access
-
Yuan Z, Zhao J, Chen Y, Yang Z, Cui W, Zheng Q. Regulating Inflammation Using Acid-Responsive Electrospun Fibrous Scaffolds for Skin Scarless Healing. Mediators of Inflammation 2014; 2014: 858045.
Open Access
-
Zhou P, Wang J, Macon A L B, Obata A, Jones J R, Kasuga T. Tailoring the delivery of therapeutic ions from bioactive scaffolds while inhibiting their apatite nucleation: a coaxial electrospinning strategy for soft tissue regeneration. RSC Adv. 2017; 7: 3992. Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech