Electrospun scaffolds have been widely used in research for biomedical applications. Both in the laboratory and for medical device, sterilization is necessary to ensure no contamination or infection during the study or when used in vivo. There are many sterilization techniques each with its advantages, limitations and effect on the medical device. Common sterilization techniques such as ultra violet radiation and steam sterilization (autoclaving) may affect the scaffold properties since they are mostly made out of polymers. Thus it is important to validate the properties of the electrospun scaffold after it has gone through the selected sterilization process. Below are some of the sterilization process and its consideration when used with electrospun scaffold.
Steam sterilization (Autoclave)
Steam sterilization is commonly found in laboratories, clinics and hospitals. Killing of the microorganism and spores involves direct contact with saturated steam under pressure. As steam is in the gaseous state, the heated steam is able to reach and effectively sterilize undulating surface and undercuts. However, inaccessible cavities or dense powder will not be suitable for this method due to barrier to penetration. This process is clean and will not leave behind any residue on the sample. However, the material used cannot be sensitive to moisture. Two common sterilization settings are temperatures of 121 oC at 30 minutes or 132 oC at 4 minutes in prevacuum sterilizer.
Electrospun nanofibers, polymers such as polylactide and polyglycolide with melting point above 121 oC may be sterilized using this method without any changes in the physical properties. However, the mechanical properties need to be checked following the "heat treatment".
To steam sterilize electrospun samples, they may be sealed in autoclavable bags prior to placing in the autoclave machine. An autoclave tape is usually stuck onto the bag and this changes color when autoclaving is completed. Wet samples or samples in liquid may be sterilized using this method.
Useful links:
Center for Disease Control and Prevention (CDC) for more information.
Click
here.
Dry heat sterilization
This form of sterilization involves exposing the sample to high temperature for substantial time duration. Typical settings are 170 oC for 60 minutes, 160 oC for 120 minutes and 150 oC for 150 minutes. Unlike steam sterilization, direct exposure of the surface to air is not necessary for killing the microorganism thus samples with enclosed cavities or powders may be sterilized using this method. Due to the high temperature and long heating duration, the samples should be able to withstand drying during the sterilization process. The long duration for this form of sterilization makes it less popular than steam sterilization. Greater changes in the mechanical properties of polymer materials can be expected due to the high temperature and heating duration. A study using polyethylene terephthalate electrospun mat showed a reduction in the elongation at break by more than 50% after steam sterilization [Duzyer et al 2013].
Useful links:
Center for Disease Control and Prevention (CDC) for more information.
Click
here.
Ultraviolet radiation
Ultraviolet (UV) radiation at wavelength between 240 - 280 nm is commonly used in laboratory to sterilize cell culture table top. Inactivation of microorganisms is through direct exposure to the UV radiation. Medical device with undulating surfaces or undercuts are not suitable for sterilization using this method as parts of it may be in the shadow and shielded away from the UV rays. Sterilization duration is usually between 20 minutes to 1 hour. Sterilization using this method is straight forward and clean with minimal preparation requirement. However, extended exposure of polymer to UV ray is known to result in reduction in the water contact angle [Duzyer et al 2013] and increase degradation of the polymer [Dong et al 2008]. In a study using electrospun polyethylene terephthalate, the changes in the mechanical property and physical property is found to be the least using UV treatment compared to ethylene oxide and steam sterilization [Duzyer et al 2013]. Mechanical, chemical and wetting properties of electrospun samples should be tested after sterilization using UV.
Useful links:
Center for Disease Control and Prevention (CDC) for more information.
Click
here.
Electron Beam and Gamma radiation
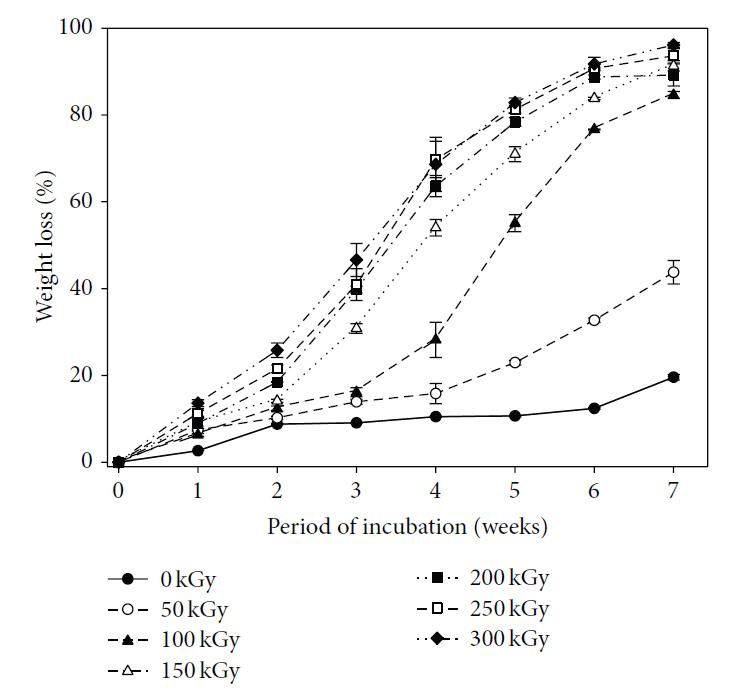
Ionizing radiation such as electron beam and gamma radiation are commonly used in the industry for sterilization of medical devices. The main reason is that the radiation is able to penetrate through packagings and medical devices thereby rendering the interior of the package and the whole device sterile. However, the main disadvantage of this method when used on electrospun polymer scaffold is the deterioration of its mechanical properties with increasing radiation dosage [Bosworth et al 2012, Nho et al 2009]. In vitro and in vivo studies using polycaprolactone have also shown increased degradation rate with higher radiation dosage with faster degradation in vivo [Nho et al 2009]. Hydrophobic material may become hydrophilic due to ionization of the material although this may be used as an advantage. Lee et al (2015) carried out several tests on the effect of electron beam irradiation dosage on electrospun poly(lactide-co-glycolide) (PLGA). With a dosage of 50 kGy, a molecular weight reduction of more than half was recorded and the corresponding weight loss at seven weeks doubles to 40%. At 100 kGy, the weight loss at seven weeks doubles again to 80%. Increasing irradiation dosage from 100 kGy to 300 kGy progressively increases the weight loss to more than 90% over the same time period. Mechanical properties such as its modulus, tensile stress and elongation at break also deteriorate with increasing E-beam dose. The most pronounced effect is at 150 kGy on the elongation at break where it was reduced from 194% at 100 kGy to just 8%. There is no observable visual difference in the fiber diameter or physical morphology throughout the tested dose from 50 kGy to 300 kGy. Yusof et al (2020) tested the influence of the electron beam on electrospun poly L-lactide acid/ carboxy-methyl starch/β-tricalcium phosphate (PLLA/CMS/β-TCP) composite nanofibers under 5, 30, and 100 kGy of irradiation dose. For its mechanical properties, even with low Irradiation of 5 kGy, there is a small reduction in its ultimate tensile strength and elongation at break. At 30 kGy, the mechanical performance were lower to irradiation at 5 kGy dose but with 100 kGy irradiation, there is a large drop in its mechanical performance. This is probably due to extensive chain scission in the molecules at high dosage which weakens the polymer composite. Interestingly, the mass loss of the composite nanofibers when immersed in PBS solution showed a slightly different pattern. At 30 days, nanofibers irradiated at 5 kGy showed the same mass loss as non-irradiated nanofibers at about 4.5% while mass losses of nanofibers irradiated at 30 kGy and 100 kGy were similar at about 7%. At 60 days, the percentage mass losses increases with higher irradiation dosage with the greatest mass loss at 10% for nanofibers irradiated at 100 kGy. As the mass loss is not proportional to soaking duration or irradiation dosage dedicated studies may be required to determine the mass loss at specific time point and irradiation dosage.
Radiation on the electrospun membrane does not always lead to deterioration of the material. Bhaskar et al (2017) compared cell response cultured on electrospun poly(ε-caprolactone) (PCL) that has been undergo gamma irradiation and ethanol submersion in an in vivo mouse tendon study. Although gamma irradiation is known to increase the hydrophilicity of PCL but not ethanol, there are no significant difference in cell migration, coverage and immune response between the two scaffolds. Rapid deposition of proteins on the scaffold surface following implantation may have made both scaffolds equally hydrophilic. It is also possible that the gamma irradiation dosage at 25 kGy is too low to cause significant change in the PCL hydrophobicity.
With electrospun poly(ε-caprolactone-co-p-dioxanone) (PCLDX) scaffolds, gamma radiation (GR) of 25 kGy at a dose rate of 0.1 Gy s-1 showed a 59% decrease in Mn [López et al 2024]. This is probably due to degradation of the polymer through chain scission by free radicals formed during GR. However, there was little effect on the hydrophobicity of the scaffold and there was only a slight drop in its crystallinity.
The influence of Gamma radiation on the electrospun material is dependent on its dosage. At lower dosage of less than 65 kGy, Augustine et al (2015) showed the ultimate tensile stress on electrospun polycaprolactone is higher than unirradiated membrane. Investigating the crystallinity of the polycaprolactone membrane showed higher crystallinity in the irradiated membrane. Cross-linking may also occur at lower dosage which contributes to higher mechanical strength. At higher dosage, chain scission may dominate and this will cause reduction in mechanical strength. As shown by Nho et al (2009), mechanical strength of electrospun polycaprolactone membrane progressively deteriorates with Gamma radiation dosage of more than 50 kGy.
Ethylene oxide
Ethylene oxide sterilization is commonly used in the industry and hospital if change to the material properties is a concern when other form of sterilization is used. Generally, the temperature for this treatment is about 37 to 63 oC, relative humidity of 40 to 80% and temperature of 37 to 63 oC. The main limitation using this method is the presence of residual ethylene oxide in the device. In particular, device with highly tortuous path with high porosity will require a longer time for sterilization (for full penetration) and subsequent aeration such that the residue falls below allowable limit. This will be applicable for sterilization of thick volume nanofibrous scaffold. Depending on the composition of the electrospun scaffold, ethylene oxide may also cause chemical reaction to the material resulting in changes to its properties [Philip Jr et al 2013]. A study using electrospun polyethylene terephthalate showed significant physical damage, increase in Young Modulus and slight drop in water contact angle after ethylene oxide sterilization [Duzyer et al 2013].
For materials with melting temperature closer to room temperature, ethylene oxide sterilization may also affect its physical property due to melting as in the case of electrospun poly[(R)-3-hydroxybutyrate]. Investigation of ethylene oxide showed no significant effect on the crystallinity, molecular weight and mechanical properties(tensile strength and modulus) of poly-L-lactide, stereocomplex polylactide, poly[(R)-3-hydroxybutyrate] [P(3HB)] and its copolymers; poly[(R)-3-hydroxybutyrate-co-5mol%-(R)-3-hydroxyhexanoate] [P(3HB-co-5mol%-3HHx)] and poly[(R)-3-hydroxybutyrate-co-7mol%-4-hydroxybutyrate] [P(3HB-co-7mol%-4HB)] [Tang et al 2010].
Sterilization of electrospun PCLDX scaffolds using EO set at 20 °C for 24 h with 17.6 g of EO showed negligible effects on Mn and hydrophobicity [Lopez et al 2024]. This is in contrast with the same scaffold sterilized using GR which showed significant drop in Mn due to chain scission.
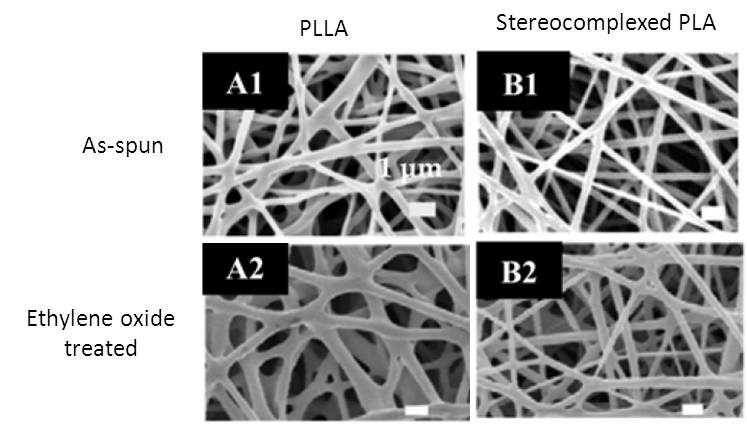
Neffe et al (2021) reported the effect of ethylene oxide (EtO) sterilization on electrospun poly(L-lactide)/poly(D-lactide) (PLLA/PDLA) core/shell nanofibers. . The sterilization condition was EtO gas (6 vol% in CO2, 1.7 bar) for 3 h at 45 °C and 75% relative humidity and degassing under pressure/vacuum cycles for 12 h. A main concern with sterilization using EtO is the possible presence of residual EtO. Their GC-MS analysis after sterilization showed no detectable EtO residuals. GPC analysis also showed no changes to the polymer composition and molecular weight. The Tg of polylactides is around 58 °C and the sterilization process using EtO was at 45°C. Sterilization was carried out in the presence of water vapor and water update into the polymer matrix may increase the polymer chain mobility and reduce the actual Tg. This may have caused the slight increase in crystallinity post sterilization. The slight increase of Young's modulus and decrease of elongation at break has been attributed to the potential small amount of interfiber bonding or slight changes to surface chemistry rather than increase in crystallinity. Biodegradable polylactides are commonly used in the construction of implantable devices. However, sterilization processes that involve heat and irradiation have been known to affect its physical properties. Therefore, EtO may have some advantages over other sterilization processes for polylactide implantable devices.
Useful links/information:
a) ISO 11135-1:2007 Sterilization of health care products -- Ethylene oxide -- Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices
b) Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
Click
here.
c) FDA Guidance document, Ethylene Oxide, Ethylene Chlorohydrin, and Ethylene Glycol. Proposed Maximum Residue Limits and Maximum Levels of Exposire.
Click
here.
Published date: 20 March 2014
Last updated: 25 June 2025
▼ Reference
-
Augustine R, Saha A, Jayachandran V P, Thomas S, Kalarikkal N. Dose-Dependent Effects of Gamma Irradiation on the Materials Properties and Cell Proliferation of Electrospun Polycaprolactone Tissue Engineering Scaffolds. International Journal of Polymeric Materials and Polymeric Biomaterials 2015; 64: 10.
-
Bhaskar P, Bosworth L A, Wong R, O'brien M A, Kriel H, Smit E, McGrouther D A, Wong J K, Cartmell S H. Cell response to sterilized electrospun poly(ε-caprolactone)
scaffolds to aid tendon regeneration in vivo. J Biomed Mater Res Part A 2017; 105A: 389.
-
Bosworth L A, Gibb A, Downes S. Gamma irradiation of electrospun poly(?-caprolactone) fibers affects material properties but not cell response. Journal of Polymer Science Part B: Polymer Physics 2012; 50: 870.
-
Dong Y, Yong T, Liao S, Chan CK, Ramakrishna S. Degradation of electrospun nanofiber scaffold by short wave length ultraviolet radiation treatment and its potential applications in tissue engineering. Tissue Eng. Part A 2008; 14: 1321.
-
Duzyer S, Koc S K, Hockenberger A, Evke E, Kahveci Z, Uguz A. Effects of Different Sterilization Methods on Polyester Surfaces. Tekstil ve Konfeksiyon 2013; 23: 319.
Open Access
-
Lee J B, Ko Y Q, Cho D, Park W H, Kim B N, Lee B C, Kang I K, Kwon O H. Modification of PLGA Nanofibrous Mats by Electron Beam Irradiation for Soft Tissue Regeneration. Journal of Nanomaterials 2015; 2015: 295807
Open Access
-
Lopez A M, Appaiah A, Berglund J, Marteleur K, Ajalloueian F, Finne-Wistrand A. Effect of ethylene oxide and gamma sterilization on surface texture of films and electrospun poly(?-caprolactone-co-p-dioxanone) (PCLDX) scaffolds. Polymer Testing 2024; 139: 108567.
https://www.sciencedirect.com/science/article/pii/S0142941824002447 Open Access.
-
Neffe AT, Zhang Q, Hommes-Schattmann PJ, Lendlein A. Ethylene oxide sterilization of electrospun poly(L-lactide)/poly(D-lactide) core/shell nanofibers. MRS Advances 2021; 6: 786.
Open Access
-
Nho Y C, Jeun J P, Lim Y M. Controlling of Degradation Effects in Radiation Processing of Polymers. IAEA, Vienna 2009, Chapter. Electrospinning of polycaprolactone and its degradation effect by radiation. P. 107.
Open Access
-
Philip Jr. E, Murthy N S, Bolikal D, Narayanan P, Kohn J, Lavelle L, Bodnar S, Pricer K. Ethylene oxide's role as a reactive agent during sterilization: effects of polymer composition and device architecture. J. Biomed. Mater. Res. B Appl. Biomater. 2013; 101: 532.
-
Tang H Y, Ishii D, Sudesh K, Yamaoka T, Iwata T. Chapter 10. Nanofibrous Scaffolds of Bio-polyesters: In Vitro and In Vivo Characterizations and Tissue Response. In Kumar A (editor), Nanofibers. ISBN 978-953-7619-86-2, pp. 438, February 2010, INTECH, Croatia.
Open Access
-
Yusof M R, Shamsudin R, Zakaria S, Azmi Abdul Hamid M, Yalcinkaya F, Abdullah Y, Yacob N. Electron-Beam Irradiation of the PLLA/CMS/β-TCP Composite Nanofibers Obtained by Electrospinning. Polymers 2020, 12, 1593.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech