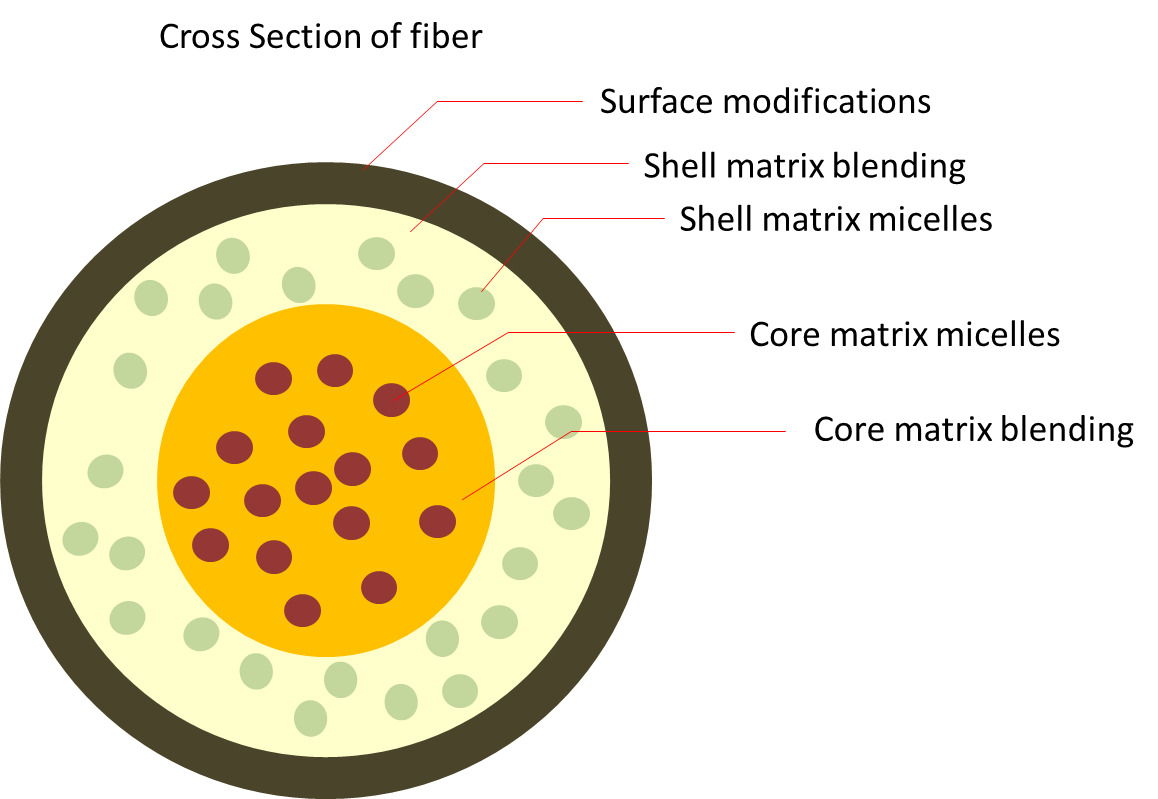
The ease and multiple ways of incorporating drugs into electrospun fibers allows for creative and tailored selection of drugs for loading with different release profiles. Core-shell electrospinning has been used to encourage controlled drug release by having a polymer matrix barrier between the drug loaded core and the surface of the fiber.
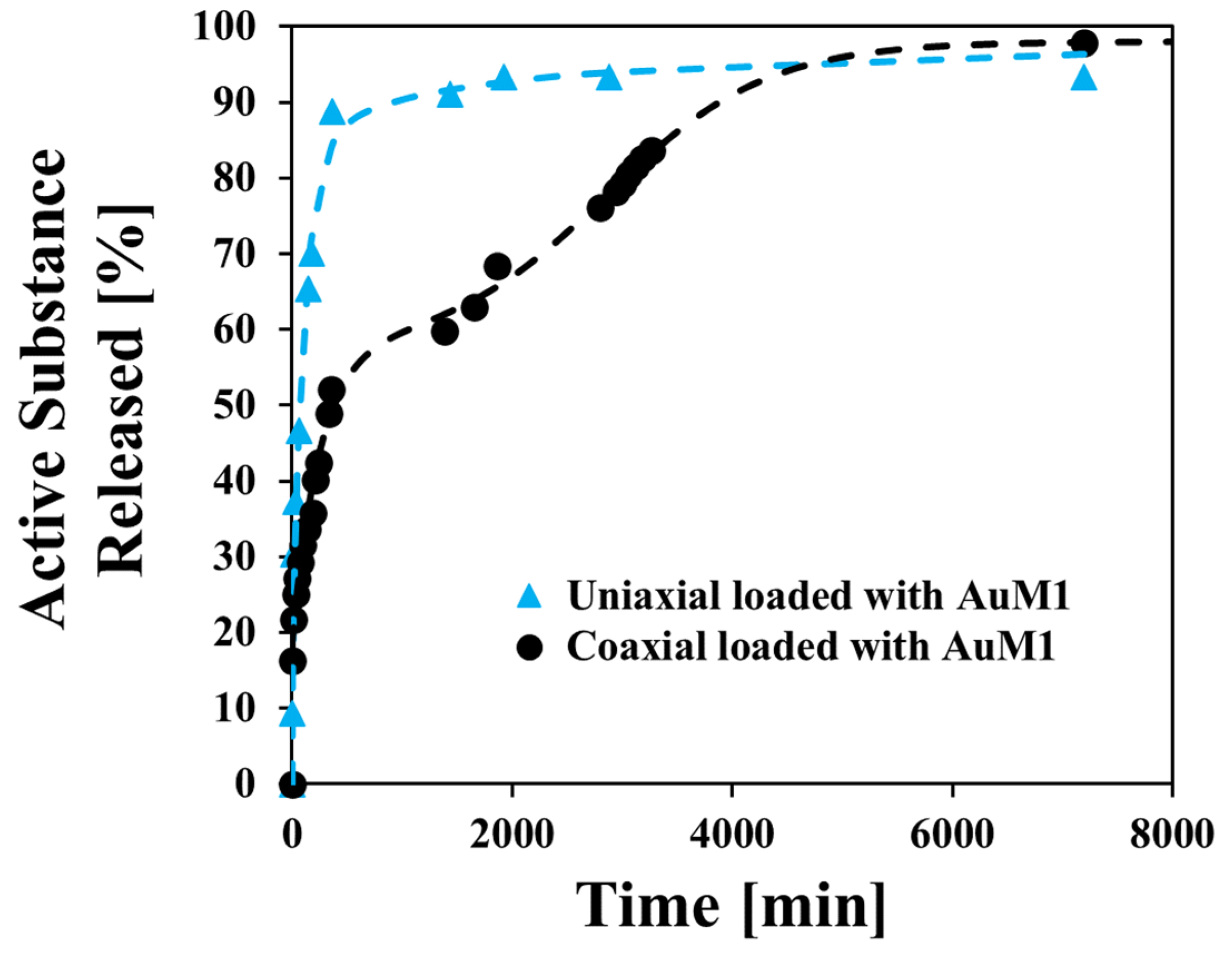
Longo et al (2024) demonstrated the effective use of core-shell electrospun fibers to reduce the release rate of antitumour agents compared to blended systems. Synthetic gold complex (AuM1) was the antitumor agent for encapsulation into a core-shell fiber with polyvinyl alcohol (PVA) shell and the AuM1 blended with polycaprolactone (PCL) core. A PBS solution was used to test the release of AuM1. Interestingly, although PVA is soluble in PBS, the core-shell fiber with PVA as the shell was able to slow the release of AuM1 to 90% over 3 days. This compared to 90% release from uniaxial fibers of AuM1 blended into PCL, in an hour. It is possible that as a uniaxial fiber, most of the AuM1 are located at or near the surface of the fiber. However, for the core-shell fiber, the hydrophilic PVA shell may discourage AuM1 from residing at the core-shell interface and instead, distributed deeper within the PCL core. The PVA in PBS was found to swell before gradually dissolving which may also hinder the release of AuM1. Looking at the release profile of AuM1 from the core-shell fibers, there was an initial faster release rate although at a slightly lower gradient than the release of AuM1 from uniaxial loaded PCL. This may be attributed to AuM1 that was initially found on the interface of the core-shell material. Swelling and dissolution of PVA in the first 16 h released about 50% of AuM1. This was followed by a much slower release of AuM1 from the remaining PCL matrix.
Additional barriers have been added to further impede the diffusion of the drugs through the use of emulsion electrospinning with the encapsulated drugs further enclosed within a core-shell fiber design. Viry et al (2012) showed that with the double encapsulation, the release rate of Levetiracetam was much slower than with core-shell fibers only. It can easily be envisioned that the core of the core-shell fibers are loaded with a drug while the emulsion within the core is given a different drug. There many opportunities for drug incorporation from the core of the fiber to its surface. Apart from modification of drug release profile within a single fiber, higher order arrangement of nanofibers and mixture may also be used to further vary the drug release behaviour.
The ability to select different materials in core-shell electrospinning makes it possible to tailor the drug release rate. Qian et al (2014) electrospun core-shell fiber with water soluble poly(vinyl pyrrolidone) (PVP) as the shell and non-water soluble ethyl cellulose (EC) as the core. The objective of this material selection is to introduce a burst release at the beginning followed by sustained release. Their experiment successfully demonstrated a dual phase release of acetaminophen (APAP, a common NSAID). For the same drug, it is sometimes desirable to have an initial burst release followed by sustained release. When the rate of drug release is slow, steps may be taken to increase the amount of surface exposed drug. Song et al (2012) introduced silica nanoparticles into the electrospun fibers loaded with drugs. The presence of the silica nanoparticles creates bumps when they are near the surface of the fibers. This has the effect of pushing more drugs to the surface of the fibers and a greater initial drug release was observed. To achieve multi-phase drug release in electrospun membrane, another method is to use multiple fiber layers with the layers made of different material.
The diffusion rate of drugs and its corresponding release profile across different materials is not the same. Instead of controlling the drug release rate within a single fiber, a simpler method is to use a mixture of fibers or different fiber layers containing the drugs. Umi-i-Zahra (2015) created a penta-layered nanofiber meshes with layers comprising of cellulose acetate (CA), polyvinyl pyrrolidone (PVP) and ethyl cellulose fibers (EC). By sandwiching a layer of drug loaded PVP between layers of CA and EC, a spike in the drug released has been demonstrated in the middle of the drug release period when the layered fibrous structure was immersed in PBS buffer solution.
Drugs may be encapsulated in nanoparticles and electrospun to create an additional barrier for the drug release. Interaction between the nanoparticles and the polymer fiber matrix may influence the dispersion of the drugs and thus its release rate. Wang et al (2011) prepared naproxen-loaded and rhodamine chitosan nanoparticles for electrospinning with polycaprolactone (PCL) solution to form separate composite fibers. The chitosan nanoparticles were found to be localized in the core region of the electrospun fibers. Comparing the release rate of rhodamine from rhodamine loaded PCL fibers and rhodamine in nanoparticles loaded PCL fibers, the former was being released at a much faster rate than the latter. This has been attributed to the incompatibility between hydrophilic rhodamine and hydrophobic PCL matrix. With chitosan nanoparticles, adhesion of rhodamine to the nanoparticles reduces its release rate from the PCL matrix.
Published date: 9 June 2015
Last updated: 07 October 2025
▼ Reference
-
Longo R, Vertuccio L, Aliberti F, Mariconda A, Raimondo M, Longo P, Guadagno L. Coaxial Electrospinning of PCL-PVA Membranes Loaded with N-Heterocyclic Gold Complex for Antitumoral Applications. Fibers. 2024; 12(12):101.
https://www.mdpi.com/2079-6439/12/12/101 Open Access.
-
Qian W, Yu D G, Li Y, Liao Y Z, Wang X, Wang L. Dual Drug Release Electrospun Core-Shell Nanofibers with Tunable Dose in the Second Phase. Int. J. Mol. Sci. 2014; 15: 774.
Open Access
-
Song B, Wu C, Chang J. Dual drug release from electrospun poly(lactic-co-glycolic acid)/mesoporous
silica nanoparticles composite mats with distinct release profiles. Acta Biomaterialia 2012; 8: 1901.
-
Um-i-Zahra S. Study of in-Vitro Drug Release Profiles of Time-controlled Penta-layered Nanofiber Meshes. International Journal of Research 2015; 2: 152.
Open Access
-
Viry L, Moulton S E, Romeo T, Suhr C, Mawad D. Emulsion-coaxial electrospinning: designing novel architectures for sustained release of highly soluble low molecular weight drugs. Journals of Materials Chemistry 2012; 22: 11347.
-
Wang Y, Qiao W, Wang B, Zhang Y, SHao P, Yin T. Electrospun composite nanofibers containing nanoparticles for the programmable release of dual drugs. Polymer Journal 2011; 43: 478.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech