▼ Reference
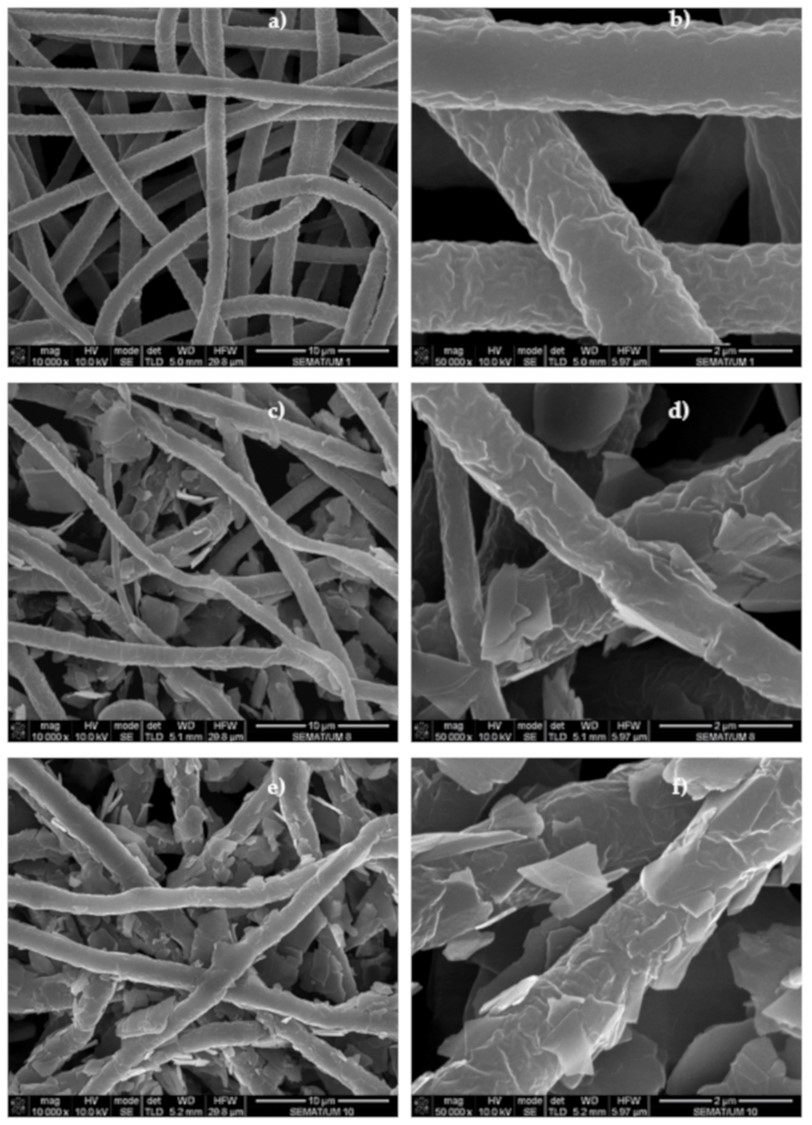
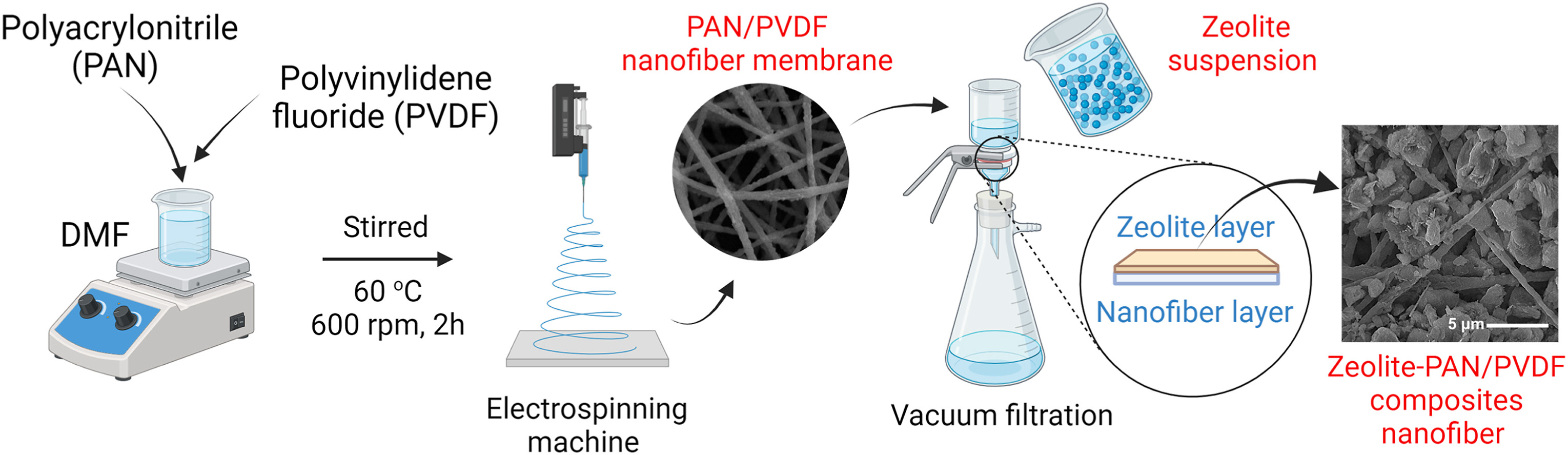
- Arif M F, Muhtar S A, Siburian C, Marpaung K D P, Yulianto N, Abdi F F, Taher T, Wasisto H S, Rianjanu A. Zeolite-PAN/PVDF composite nanofiber membranes for highly efficient and selective removal of cationic dyes from wastewater. Case Studies in Chemical and Environmental Engineering 2024; 10: 100806. https://www.sciencedirect.com/science/article/pii/S2666016424002007 Open Access
- Dong Q, Wang G, Hu H, Yang J, Qian B, Ling Z, Qiu J. Ultrasound-assisted preparation of electrospun carbon nano?ber/graphene composite electrode for supercapacitors. Journal of Power Sources 2013; 243: 350.
- Francavilla P, Ferreira DP, Araújo JC, Fangueiro R. Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets. Applied Sciences. 2021; 11(3):1124. Open Access
- Jaworek A, Krupa A, Lackowski M, Sobczyk A T, Czech T, Ramakrishna S, Sundarraja S, Pliszka D. Nanocomposite fabric formation by electrospinning and electrospraying technologies. Journal of Electrostatics 2009; 67: 435.
- Kang J, Chen L, Sukigara S. Preparation and Characterization of Electrospun Polycaprolactone Nanofiber Webs Containing Water-soluble Eggshell Membrane and Catechin. Journal of Fiber Bioengineering & Informatics. 2012; 5: 217. Open Access
- Kang M, Jin H J. Electrically conducting electrospun silk membranes fabricated by adsorption of carbon nanotubes. Colloid Polym Sci 2007; 285: 1163.
- Phan D N, Choi H Y, Oh S G, Kim M, Lee H. Fabrication of ZnO Nanoparticle-Decorated Nanofiber Mat with High Uniformity Protected by Constructing Tri-Layer Structure. Polymers 2020; 12(9): 1859 Open Access
- Teno J, Pardo-Figuerez M, Hummel N, Bonin V, Fusco A, Ricci C, Donnarumma G, Coltelli M-B, Danti S, Lagaron JM. Preliminary Studies on an Innovative Bioactive Skin Soluble Beauty Mask Made by Combining Electrospinning and Dry Powder Impregnation. Cosmetics. 2020; 7(4):96. Open Access
- Trejo N K, Frey M. A comparative study on electrosprayed, layer-by-layer, and chemically grafted nanomembranes loaded with iron oxide nanoparticles. J. Appl. Polym. Sci. 2015; 132: 42657.
- Xuyen N T, Kim T H, Geng H Z, Lee I H, Kim K K, Lee Y H. Three-dimensional architecture of carbon nanotube-anchored polymer nano?ber composite. J. Mater. Chem. 2009; 19: 7822.
- Zhang Y, Lee M W, An S, Sinha-Ray S, Khansari S, Joshi B, Hong S, Hong J H, Kim J J, Pourdeyhimi B, Yoon S S, Yarin A L. Antibacterial activity of photocatalytic electrospun titania nano?ber mats and solution-blown soy protein nano?ber mats decorated with silver nanoparticles. Catalysis Communications 2013; 34: 35.
- Zheng YY, Miao JJ, Maeda N, Frey D, Linhardt R J, Simmons T J. Uniform nanoparticle coating of cellulose fibers during wet electrospinning. J. Mater. Chem. 2014; 2:15029.
▼ Credit and Acknowledgement
Author
Wee-Eong TEO View profile
Email: weeeong@yahoo.com
 ElectrospinTech
ElectrospinTech