▼ Reference
- Cho J S, Park J S, Kang Y C. Preparation of Hollow Fe2O3 Nanorods and Nanospheres by Nanoscale Kirkendall Diffusion, and Their Electrochemical Properties for Use in Lithium-Ion Batteries. Scientific Reports 2016; 6: 38933. Open Access
- Dong Q, Wang G, Hu H, Yang J, Qian B, Ling Z, Qiu J. Ultrasound-assisted preparation of electrospun carbon nanofiber/graphene composite electrode for supercapacitors. Journal of Power Sources 2013; 243: 350.
- Fan C, Sun F, Wang X, Huang Z, Keshvardoostchokami M, Kumar P, Bo Liu B. Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties. Nanomaterials 2019; 9(9): 1277 Open Access
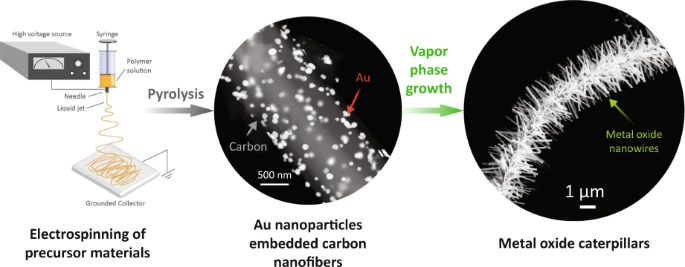
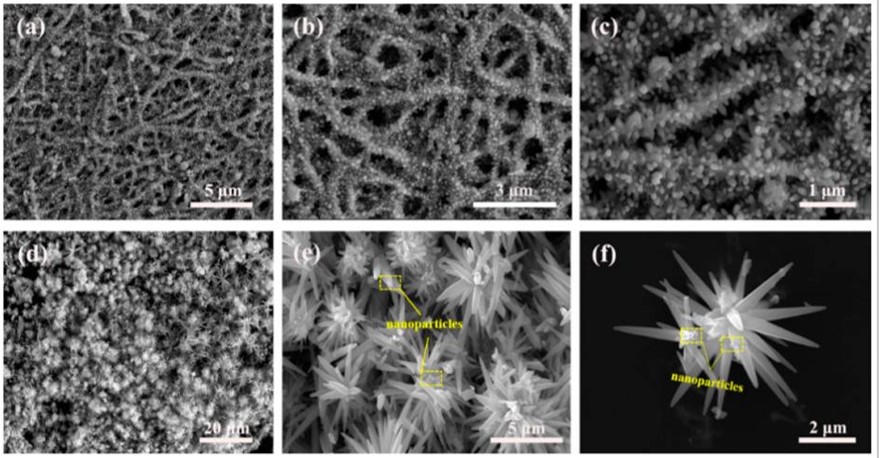
- Islam M, Dolle C, Sadaf A, Weidler P G, Sharma B, Eggeler Y M, Mager D, Korvink J G. Electrospun carbon nanofibre-assisted patterning of metal oxide nanostructures. Microsyst Nanoeng 2022; 8: 71. Open Access
- Jaworek A, Krupa A, Lackowski M, Sobczyk A T, Czech T, Ramakrishna S, Sundarraja S, Pliszka D. Nanocomposite fabric formation by electrospinning and electrospraying technologies. Journal of Electrostatics 2009; 67: 435.
- Jia B B, Wang J N, Wu J, Li C J. "Flower-Like" PA6@Mg(OH)2 electrospun nanofibers with Cr (VI)-removal capacity. Chemical Engineering Journal 2014; 254: 98.
- Lai C, Guo Q, Wu X F, Reneker D H, Hou H. Growth of carbon nanostructures on carbonized electrospun nanofibers with palladium nanoparticles. Nanotechnology 2008; 19: 195303.
- Lu F, Wang J, Chang Z, Zeng J. Uniform deposition of Ag nanoparticles on ZnO nanorod arrays grown on polyimide/Ag nanofibers by electrospinning, hydrothermal, and photoreduction processes. Materials & Design 2019; 181: 108069 Open Access
- Meng F, Zhan Y, Lei Y, Zhao R, Xu M, Liu X. Rose thorns-like polymer micro/nanofibers via electrospinning and controlled temperature-induced self-assembly. European Polymer Journal 2011; 47: 1563.
- Meng X, Shin D W, Yu S M, Jung J H, Kim H I, Lee H M, Han Y H, Bhoraskar V, Yoo J B. Growth of hierarchical TiO2 nanostructures on anatase nanofibers and their application in photocatalytic activity. CrysEngComm 2011; 13: 3021.
- Meng Z X, Li H F, Sun Z Z, Zheng W, Zheng Y F. Fabrication of mineralized electrospun PLGA and PLGA/gelatin nanofibers and their potential in bone tissue engineering. Materials Science and Engineering 33; 2013: 699.
- Kang J, Chen L, Sukigara S. Preparation and Characterization of Electrospun Polycaprolactone Nanofiber Webs Containing Water-soluble Eggshell Membrane and Catechin. Journal of Fiber Bioengineering & Informatics. 2012; 5: 217.
- Kang M, Jin H J. Electrically conducting electrospun silk membranes fabricated by adsorption of carbon nanotubes. Colloid Polym Sci 2007; 285: 1163.
- Ochanda F O, Rajukada S, Barnett M R. Controlled Synthesis of TiO2 Hierarchical Nanofibre Structures via Electrospinning and Solvothermal Processes: Photocatalytic Activity for Degradation of Methylene Blue. Nanomater. Nanotechnol. 2012; 2: 1. Open Access
- Virovska D, Paneva D, Manolova N, Rashkov I, Karashanova D. Electrospinning/electrospraying vs. electrospinning: A comparativestudy on the design of poly(l-lactide)/zinc oxide non-woven textile. Applied Surface Science 2014; 311: 842.
- Wang B, Li B, Xiong J, Li C Y. Hierarchically Ordered Polymer Nanofibers via Electrospinning and Controlled Polymer Crystallization. Macromolecules 2008; 41: 9516.
- Xuyen N T, Kim T H, Geng H Z, Lee I H, Kim K K, Lee Y H. Three-dimensional architecture of carbon nanotube-anchored polymer nano?ber composite. J. Mater. Chem. 2009; 19: 7822.
- Zhang Y, Lee M W, An S, Sinha-Ray S, Khansari S, Joshi B, Hong S, Hong J H, Kim J J, Pourdeyhimi B, Yoon S S, Yarin A L. Antibacterial activity of photocatalytic electrospun titania nanofiber mats and solution-blown soy protein nanofiber mats decorated with silver nanoparticles. Catalysis Communications 2013; 34: 35.
- Zhao K, Teng L, Tang Y, Chen X. Branched titaniumoxide/vanadium oxide composite nanofibers formed by electrospinning and dipping in vanadium sol. Ceramics International 2014; 40: 15335.
- Zheng YY, Miao JJ, Maeda N, Frey D, Linhardt R J, Simmons T J. Uniform nanoparticle coating of cellulose fibers during wet electrospinning. J. Mater. Chem. 2014; 2:15029.
▼ Credit and Acknowledgement
Author
Wee-Eong TEO View profile
Email: weeeong@yahoo.com
 ElectrospinTech
ElectrospinTech