A continuous, seamless and porous tube comprising of nanofibers can be easily constructed using electrospinning. With a wide selection of materials suitable for electrospinning, a vascular graft with tailored characteristics can be constructed. Currently, most investigations of electrospun vascular grafts are targeted at small diameter vessels that are less than 2 mm in diameter. Such small diameter vessels are more susceptible to thrombosis and intimal hyperplasia. Some of the requirements for ideal vascular graft are:
- Biocompatibility
- Non-thrombogenic
- Good suturability
- Long term compliancy
- Long term patency
Electrospun materials have been demonstrated in many in vitro studies to be compatible for endothelial cell adhesion and proliferation. In vivo studies have also been carried out to determine its functional performance. Tillman et al (2009) tested the stability of electrospun polycaprolactone-collagen vascular graft in a rabbit infrarenal aorta model. After one month, the graft was able to maintain its morphological configuration and patency was confirmed in seven out of eight animals although endothelial cells were absent from the lumen. The implant showed good biocompatibility with no inflammatory infiltrate. Examining the circumferential tensile strength showed a dropped to half of its pre-implant value, probably due to the degradation of the material. In a study by He et al (2009) using poly(lactide-co-caprolactone) conduits implanted in a rabbits inferior superficial epigastric veins model, the tube showed patency for 7 weeks although there was also no sign of endothelial cell migration into its lumen. Electrospun polycaprolactone vascular graft also demonstrated patency (78%) with evidence of endothelialization after 8 weeks in a rat carotid artery model [Kuwabara et al 2012].Gao et al (2016) implanted 1 diameter (inner) electrospun resorbable, amino acid-based poly(ester urea) (PEU) tube with different wall thickness into the abdominal infra-renal aortic grafts of a severe combined immune deficient/beige mouse model and evaluated for vessel remodeling over one year. While the 250 µm wall thickness showed well-circumscribed neovessels with an endothelial inner lining and a neointima containing smooth muscle cells, the 350 µm wall thickness suffer from intimal hyperplasia and occlusion. The difference in performance may be due to the mechanical behavior difference between them since the material and surface characteristics were the same.
Depending on the design consideration for the vascular graft, some researchers may prefer cell infiltration into the graft to ensure good integration while others may prefer one that obstruct cell infiltration to avoid risk of blood leakage or cell infiltration into the graft lumen. For supporters of the former, good infiltration of cells within the graft allows better host tissue integration over the long term. However, infiltration of the cells should not lead to occlusion of the graft lumen. While the graft may allow cell infiltration, there must not be any blood leakage after graft implantation. Bergmeister et al (2013) compared the performance of electrospun polyurethane fibrous vascular graft with diameter of 0.9 µm (porosity 53%) and 1.0 µm in vivo (porosity 80%). The in vivo tests were carried out in Sprague Dawley rats infrarenal aorta. After six months of implantation, their tests showed that the finer fiber graft restricted host cells to the outer one-third of the graft while considerable infiltration of cells were found throughout the larger fiber diameter graft. Long term survival of the cells were also found to be better in the larger fiber diameter graft with collagen type 1 found within the spaces in the graft. Importantly, there are no blood leakage on both fine and larger fiber diameter graft.
Drugs known to reduce intimal hyperplasia such as paclitaxel (PTX) may be introduced to electrospun graft to maintain its patency and flow. Innocente et al (2009) demonstrated the effect of incorporating PTX to electrospun polycaprolactone (PCL) vascular graft in a rat aorta model. Cellular infiltration and migration of endothelial cells from both anastomoses was evident in PCL without PTX at 3 weeks but none was observed in the PCL graft with PTX. At 24 weeks, confluent endothelial coverage was found in both grafts but neointima formation in the graft with PTX was less than half that without PTX. Longer term study is required to determine if the delayed cellular infiltration helps to prevent intimal hyperplasia. Hashi et al (2010) bonded Hirudin to electrospun PLLA fibers with the help of di-amino polyethylene glycol (PEG). The presence of PEG and Hirudin-PEG added to PLLA was able to inhibit platelet adhesion on the fiber surface as compared to pure PLLA. In vivo study showed that the Hirudin-PEG grafted PLLA was able to maintain patency at 6 months. Their study also showed endothelialisation on the luminal surface with complete endothelial coverage at 1 month and 6 months. More than one drugs may be incorporated into electrospun fibers to improve the performance of vascular graft implant. Gao et al (2018) used layer-by-layer (LBL) assembly on electrospun polycaprolactone (PCL) fibers (fiber diameter of 6.5 µm) to build a vascular graft with in situ nitric oxide (NO) generation capability and heparin. In situ nitric oxide (NO) generation comes from selenium-containing catalyst-organoselenium modified polyethyleneimine (SePEI) which were introduced through layer-by-layer assembly. SePEI could decompose S-nitrosothiols from peripheral blood to NO. For the LBL assembly, the electrospun PCL scaffold was alternately dipped in polycation (SePEI) and polyanion (heparin) with a oolydiallyldimethylammonium chloride (PDDA)/heparin as a precursor layer. In vivo study using a rat abdominal aorta model showed more complete endothelialization and almost no fibers were observed in the PCL-(SePEI/Hep)10 grafts after one month compared to only a small amount of endothelium covering the anastomosis site of PCL grafts. However, almost complete endothelialization of both kinds of grafts was achieved at two months. Intimal hyperplasia (IH) were observed on both grafts after two weeks with IH in the PCL grafts (a layer of about 10 cell sheets, 76 µm) about twice as thick as PCL-(SePEI/Hep)10.
The ability to maintain patency in the vascular graft is a challenge in long term arterial model. Hong et al (2009) reported just 40% patency for poly(ester urethane)urea (PEUU) electrospun grafts and 67% patency for non-thrombogenic poly(2-methacryloyloxyethyl phosphorylcholine-co-methacryloyloxyethyl butylurethane) (PMBU)/PEUU blended electrospun grafts after 8 weeks implant in a rat infrarenal aorta model. The relatively better performing PMBU/PEUU blend showed continuous endothelium in the lumen with no thrombogenic deposition and intimal hyperplasia but electrospun PEUU grafts showed occluded lumen. To further reduce thrombogenesis, non-thrombogenic phospholipid copolymer has been grafted to the surface of electrospun PEUU instead of blending. This resulted in a much better performance of 92% patency at 8 weeks in the same animal model with the same continuous endothelium lining observed. Mechanical performance showed less compliance but similar stress and stretch value compared to rat aortas at 12 weeks [Soletti et al 2011]. Long term patency of the graft depends on full endothelialization of the graft as this will prevent intimal hyperplasia.
In native blood vessel, a layer endothelial cells lines the wall of the lumen and this is thought to prevent thrombus formation. In vitro studies have shown that a layer of mesenchymal stem cells (MSC) or endothelial cells (ECs) were able to lower platelet adhesion/aggregation while smooth muscle cells (SMCs) performed poorly [Hashi et al 2007]. Similarly, having a layer of MSCs or ECs in the lumen of graft may be the solution for long term patency of the graft. Hashi et al (2007) seeded mesenchymal stem cells (MSC) onto a sheet of electrospun poly(L-lactic acid) (PLLA) scaffold and physically roll into a tube before implantation into a rat common carotid artery. After 60 days, the MSC-seeded vascular grafts showed little intimal thickening in contrast to intimal thickening in the lumen of acellular grafts. In contrast, implanted electrospun poly(L-lactic acid) (PLLA) acellular scaffold in a rat common carotid artery showed significant intimal thickening in the lumen after 60 days [Hashi et al 2007].
Niu et al (2021) constructed a bilayered electrospun tubular vascular scaffold with the inner layer made of electrospun hyaluronic acid (HA) fibers and the outer layer made of electrospun collagen fibers. The bilayered vascular scaffold was cross-linked with glutaraldehyde vapor. To mimic native vascular graft, the inner lumen of the scaffold was seeded with endothelial cells (EC) and the outer surface was seeded with smooth muscle cells (SMC). Platelet adhesion test by exposing the scaffolds to arterial flow showed that bare scaffolds without endothelial cells coverage resulted in numerous platelets adhesion. In contrast, scaffolds with endothelial cells coverage in the lumen had significantly less platelets adhesion. In vivo tests on a rabbit carotid artery model showed that the bilayered scaffold with EC and SMC seeded was able to maintain a mechanical strength close to that of native artery after 6 weeks. In the same period, all cellularized bilayered scaffolds remained patent and their lumen diameter unchanged. Therefore, this cellularized tubular HA/collagen nanofibrous scaffold has the potential to be used as a vascular graft.
Rapid endothelialization of the graft may be a better long term strategy than to prevent any cell infiltration. Peptides that selectively favor endothelial cell adhesion and migration over smooth muscle cells may be added to the graft. Kuwabara et al (2012) selected peptide CAG (cysteine-alanine-glycine), which demonstrated this ability for blending into polycaprolactone electrospun vascular graft. In a rat carotid artery model, electrospun polycaprolactone vascular graft with the peptide demonstrated higher degree of endothelialization and less expression of smooth muscle cells from 6 to 8 weeks. Filipe et al (2018) showed excellent result from electrospun Bombyx mori silk grafts implanted in an rat infrarenal abdominal aorta model. The implanted acellular graft showed full endothelialization by 12 weeks with near complete endothelialization at week 6. Hyperplasia was also found to stabilize by 6 weeks with reduction of hyperplasia to 27% at 12 weeks onwards. Graft survival rate at 24 weeks was 95% for the silk grafts compared to 73% for ePTFE graft.
The absence of an extracellular matrix (ECM) in synthetic grafts may limit the integration of the graft with the host body. One method of introducing ECM into the graft is by first implanting the scaffold subcutaneously and using the body as the bioreactor for cell infiltration and deposition of ECM. Su et al (2022) demonstrated this method using a rabbit carotid artery model and electrospun Poly (L-lactic-co-ε-caprolactone) (PLCL) /polyurethane (PU) small diameter vascular graft. In this study, PLCL/PU graft was first implanted into the abdominal subcutaneous area for 4 weeks. Prior to implantation, a silicone tube was placed in the lumen of electrospun PLCL/PU graft to prevent deposition and occlusion of the graft. After 4 weeks of embedding, the PLCL/PU graft with the silicone tube was removed. The silicone tube was extracted from the PLCL/PU graft and the graft was subsequently stored in 75% ethanol solution to kill the cells. This PLCL/PU biotube was covered with collagen on the inner and outer surfaces. The efficacy of the biotube and pure PLCL/PU graft was tested in a rabbit carotid artery model. The PLCL/PU grafts group were all occlusive within 4 weeks while the biotubes group was able to maintain a 62.5% primary patency after 12 weeks of implantation. The biotubes after 12 weeks of implantation showed smooth muscle cells (SMCs) arranged in layers while endothelial cells (EC) were present in the luminal surface although less than the native rabbit aorta EC count. Larger count of SMCs in the biotube compared to native rabbit aorta cell count may suggest rising risk of intimal hyperplasia although longer studies would be needed to determine this.
In vitro studies have shown that aligned nanofibers are able to stimulate endothelial cells alignment. However, there are few in vivo investigation on the effect of fiber alignment vascular graft performance. There is at least one study which showed that electrospun polyurethane nanofibers which are longitudinally aligned in the lumen was able to maintain 100% patency with no thrombus formation compared to just 29% patency in vascular graft with smooth topography, after implanting for 15 min into rat abdominal aorta [Liu et al 2013]. Although this preliminary study shows a benefit of vascular graft topography, more studies are needed to verify this.
For greater stability in the vascular graft property, most materials used are often non-biodegradable. While there may be short term stability in the graft property, recognition of the graft as a foreign body will stimulate the surrounding host tissue to wall off the vascular graft. Over a long duration, the functioning of the vascular graft may start to deteriorate if fibroid tissues formed are dense enough to impair the graft performance which may require the removal and replacement of the implant. With the promising short term in vivo results, clinical trials using electrospun vascular graft have been conducted with duration of up to a year. Commercially available vascular graft, AVflo, is designed for use as a vascular access graft solution to hemodialysis patients. Clinical trial report for study up to a year has shown that the graft patencies were 50% and access thrombosis of 42% [Karatepe et al 2013]. This result is similar to other commercially available vascular grafts.
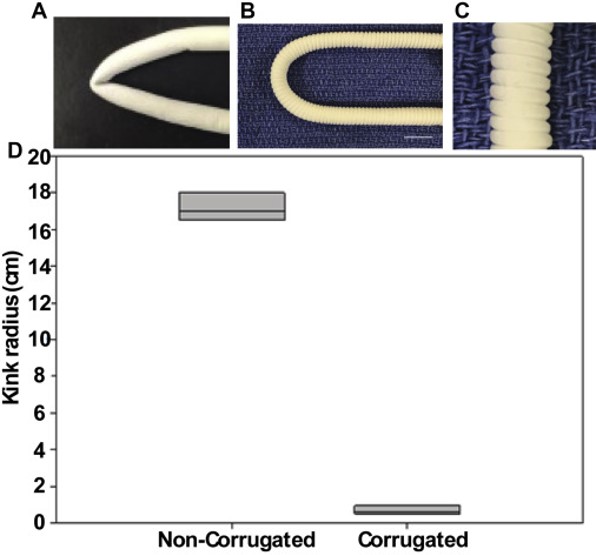
In clinical application of vascular graft a physical factor to consider is the non-kinking after it has been implanted. Commercially available grafts are mostly rigid and easily kink when implanted. Matsushita et al (2020) formed corrugation on a tube made of co-electrospun electrospun poly(L-lactide-co-caprolactone)/poly-ε-caprolactone (PCL/PLCL) nanofibers so that the corrugated tube is able to resist kinking. Such corrugation was formed by wrapping a monofilament around the electrospun tube, longitudinally compressed and allowing it to set for 24 h before the tube was relaxed and the filament removed. The electrospun graft was implanted in a sheep model. There was no significant kinking in the grafts and endothelial cells were observed in the grafts 1 month after the surgeries.
Published date: 05 August 2014
Last updated: 26 September 2023
▼ Reference
-
Bergmeister H, Schreiber C, Grasl C, Walter I, Plasenzotti R, Stoiber M, Bernhard D, Schima H. Healing characteristics of electrospun polyurethane grafts with various porosities. Acta Biomaterialia 2013; 9: 6032.
-
Filipe E C, Santos M, Hung J, Lee B S L, MPhil N Y, Chan A H P, Ng M K C, Rnjak-Kovacina J, Wise S G. Rapid Endothelialization of Off-the-Shelf Small Diameter Silk Vascular Grafts. JACC: Basic to Translational Science 2018; 3: 38
Open Access
-
Gao J, Jiang L, Liang Q, Shi J, Hou D, Tang D, Chen S, Kong D, Wang S. The grafts modified by heparinization and catalytic nitric oxide generation used for vascular implantation in rats. Regenerative Biomaterials 2018; rby003,
Open Access
-
Gao Y, Yi T, Shinoka T, Lee Y U, Reneker D H, Breuer C K, Becker M L. Pilot Mouse Study of 1 mm Inner Diameter (ID) Vascular Graft Using Electrospun Poly(ester urea) Nanofibers. Advanced Healthcare Materials 2016. Article in press
-
Hashi C K, Zhu Y, Yang G Y, Young W L, Hsiao B S, Wang K, Chu B, Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. PNAS 2007; 104: 11915.
Open Access
-
Hashi C K, Derugin N, Janairo R R R, Lee R, Schultz D, Lotz J, Li S. Antithrombogenic Modification of Small-Diameter Microfibrous Vascular Grafts. Arterioscler Thromb Vac Biol 2010; 30: 1621.
Open Access
-
He W, Ma Z, Teo W E, Dong Y X, Robless P A, Lim T C, Ramakrishna S. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. J Biomed Mater Res A 2009; 90: 205.
-
Hong Y, Ye S H, Nieponice A, Soletti L, Vorp D A, Wagner W R. A small diameter, fibrous vascular conduit generated from a poly(ester urethane)urea and phospholipid polymer blend. Biomaterials 2009; 30: 2457.
-
Innocente F, Mandracchia D, Pektok E, Nottelet B, Tille J C, Valence S, Faggian G, Mazzucco A, Kalangos A, Gurny R, Moeller M, Walpoth B H. Paclitaxel-Eluting Biodegradable Synthetic Vascular Prostheses. A Step Towards Reduction of Neointima Formation? Circulation 2009; 120: S37.
Open Access
-
Karatepe C, Altinay L, Yetim T D, Dagli C, Sursun S. A novel electrospun nano-fabric graft allows early cannulation access and reduces exposure to central venous catheters. J Vas Access 2013; 14: 273.
-
Kuwabara F, Narita Y, Yamawaki-Ogata A, Kanie K, Kato R, Satake M, Kaneko H, Oshima H, Usui A, Ueda Y. Novel Small-Caliber Vascular Grafts With Trimeric Peptide for Acceleration of Endothelialization. Ann Thorac Surg 2012; 93: 156.
-
Liu R, Qin Y, Wang H, Zhao Y, Hu Z, Wang S. The in vivo blood compatibility of bio-inspired small diameter vascular graft: effect of submicron longitudinally aligned topography. BMC Cardiovascular Disorders 2013; 13: 79.
Open Access
-
Matsushita H, Inoue T, Abdollahi S, Yeung E, Ong C S, Lui C, Pitaktong I, Nelson K, Johnson J, Hibino N. Corrugated nanofiber tissue-engineered vascular graft to prevent kinking for arteriovenous shunts in an ovine model. JVS: Vascular Science 2020; 1: 100.
Open Access
-
Niu Y, Galluzzi M, Fu M, Hu J, Xia H. In vivo performance of electrospun tubular hyaluronic acid/collagen nanofibrous scaffolds for vascular reconstruction in the rabbit model. J Nanobiotechnol 2021; 19: 349.
Open Access
-
Soletti L, Nieponice A, Hong Y, Ye S H, Stankus J J, Wagner W R, Vorp D A. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J Biomed Mater Res Part A 2011; 96A: 448.
-
Su Z, Xing Y, Wang F, Xu Z, Gu Y. Biological small-calibre tissue engineered blood vessels developed by electrospinning and in-body tissue architecture. J Mater Sci: Mater Med 2022; 33: 67.
Open Access
-
Tillman B W, Yazdani S K, Lee S J, Geary R L, Atala A, Yoo J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009; 30: 583
▲ Close list
 ElectrospinTech
ElectrospinTech