Electrospinning is a very versatile technique for producing a layer of nanofibers. For the construction of small diameter vascular grafts, the nanofibers may be coated on a rotating rod and removed to form a tubular scaffold. An alternative method would be to first electrospin a membrane and then roll the membrane into a tube. Diameters from as small as a few millimeters to a couple of centimeters can easily be constructed using electrospinning. Electrospinning has also been used with other techniques to create more robust tubes.
For vascular grafts, the mechanical strength of electrospun tubes may not be adequate. To overcome this limitation of electrospun tubes, Liu et al (2015) constructed a tri-layer tube with a micro-imprinted poly-p-dioxanone (PPDO) middle layer sandwiched between electrospun chitosan/polyvinyl alcohol layers. The PPDO layer provides the mechanical support while the electrospun layers provide a favourable environment for cell proliferation. Comparison of the mechanical properties showed significantly better tensile strength and radial strength at 19.8 MPa and 128 KPa respectively for the tri-layer tube with comparable specimen thickness to electrospun only tube.
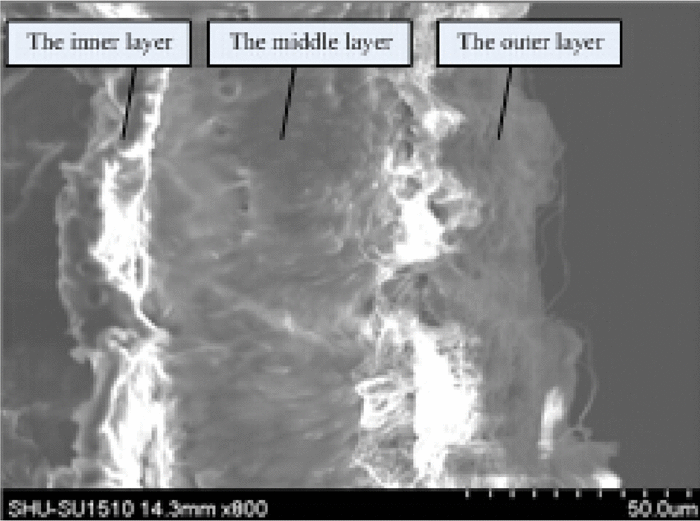
Wu et al (2023) used a combination of electrospun core-shell poly(L-lactide-co-ε-caprolactone) (PLCL) core/ collagen (Col) shell fibers and electrospun solid PLCL fibers to form a tri-layered tubular scaffold. The inner and outermost layers were the core-shell PLCL/Col fibers and the intermediate layer was made of PLCL fibers to provide the mechanical strength. To enable the removal of the electrospun tubular structure, Pluronic F127 hydrogel was used to coat the surface of the stainless steel rod prior to electrospinning. After the desired layers of nanofibers were deposited on the coated rod, the rod with the nanofibers were chilled to between 0 to 4°C to liquefy the F127 so that the electrospun scaffold can be removed from the rod easily. Residual amount of F127 may be removed using absolute ethanol. In vivo study was carried out by implanting the tubular scaffold into the pig femoral artery. The lumen of the tubular scaffold was found to be covered by a single layer of cells connected by extracellular matrix (ECM) after 4 weeks. More cells were found on the ends of the scaffold compared to the middle probably due to the slow migration of the cells from the connected host tissues.
Hybrid tubes have also been created for the purpose of introducing different topography to the tube. To create longitudinal micropatterning on the lumen of a hybrid tube, Uttayarat et al (2010) first constructed a microgroove template using poly(dimethylsiloxane). This microgroove template is subsequently rolled onto a rod and secured using nail polish. Polyurethane solution was spun cast onto the slowly rotating rod with the template under a high-intensity halogen lamp. This accelerated drying of the polyurethane solution on the template. Electrospinning of polyurethane solution was carried out immediately after the spin casting process. Semi-dried spin casted polyurethane may favor good adhesion with the electrospun polyurethane fibers.
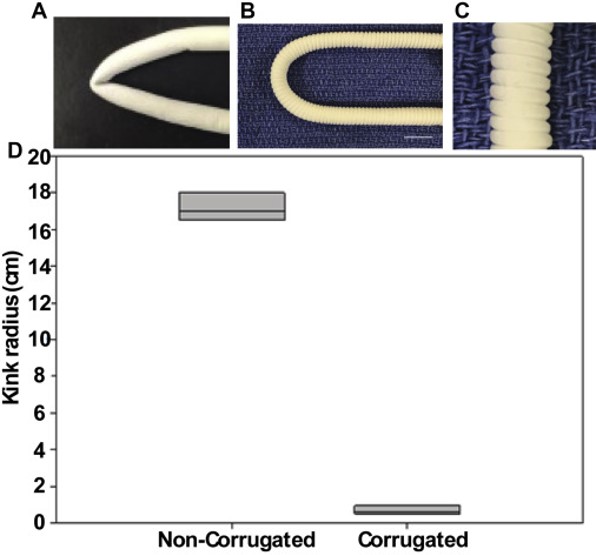
In clinical application of vascular graft a physical factor to consider is the non-kinking after it has been implanted. Commercially available grafts are mostly rigid and easily kink when implanted. Matsushita et al (2020) formed corrugation on a tube made of co-electrospun electrospun poly(L-lactide-co-caprolactone)/poly-ε-caprolactone (PCL/PLCL) nanofibers so that the corrugated tube is able to resist kinking. Such corrugation was formed by wrapping a monofilament around the electrospun tube, longitudinally compressed and allowing it to set for 24 h before the tube was relaxed and the filament removed. The electrospun graft was implanted in a sheep model. There was no significant kinking in the grafts and endothelial cells were observed in the grafts 1 month after the surgeries.
Most vascular grafts are implanted and fixed onto the injury site by means of suturing. Therefore, a clinical requirement of a vascular graft is its suture retention strength. Meng et al (2019) investigated the suture retention strength of electrospun poly(L-lactide-co-caprolactone) P(LLA-CL) vascular grafts based on its construction, wall thickness and number of stitches. With graft wall thickness greater than 0.24 mm, the electrospun scaffold was able to meet the suture retention strength even for axially oriented fibers. Scaffolds with randomly and circumferentially oriented fibers have greater suture retention strength than axially oriented fibers therefore a 0.24 mm wall thickness would provide sufficient strength for any fiber orientation.
Electrospun materials have been demonstrated in many in vitro studies to be compatible for endothelial cell adhesion and proliferation. In vivo studies have also been carried out to determine its functional performance. Tillman et al (2009) tested the stability of electrospun polycaprolactone-collagen vascular graft in a rabbit infrarenal aorta model. After one month, the graft was able to maintain its morphological configuration and patency was confirmed in seven out of eight animals although endothelial cells were absent from the lumen. The implant showed good biocompatibility with no inflammatory infiltrate. Examining the circumferential tensile strength showed a drop to half of its pre-implant value, probably due to the degradation of the material. In a study by He et al (2009) using poly(lactide-co-caprolactone) conduits implanted in a rabbits inferior superficial epigastric veins model, the tube showed patency for 7 weeks although there was also no sign of endothelial cell migration into its lumen. Electrospun polycaprolactone vascular graft also demonstrated patency (78%) with evidence of endothelialization after 8 weeks in a rat carotid artery model [Kuwabara et al 2012].Gao et al (2016) implanted 1 diameter (inner) electrospun resorbable, amino acid-based poly(ester urea) (PEU) tube with different wall thickness into the abdominal infra-renal aortic grafts of a severe combined immune deficient/beige mouse model and evaluated for vessel remodeling over one year. While the 250 µm wall thickness showed well-circumscribed neovessels with an endothelial inner lining and a neointima containing smooth muscle cells, the 350 µm wall thickness suffered from intimal hyperplasia and occlusion. The difference in performance may be due to the mechanical behavior difference between them since the material and surface characteristics were the same.
Beyond small diameter vascular grafts, electrospinning may be used to construct vascular patches. Mayoral et al (2022) used a combination of 3D printing and electrospinning for the production of the vascular patch. 3D printing has the advantage of forming a scaffold with a definite shape. Mayoral et al (2022) built the shape of the patch based on the CT scan of a patient's aorta. Fused deposition method with polycaprolactone (PCL) was used to form the patch with four-layered grid pattern design. To deposit electrospun fibers on the 3D printed patch, the collector was given a negative high voltage to direct the electrospinning jet to the collector while a positive high voltage was applied to the nozzle for electrospinning. For the surface of the patch that faces the adventitia, a lower density of electrospun fibers were deposited and for the surface that faces the intima, a higher density of electrospun fibers were deposited. The lower fiber density surface would have greater pore size to allow seeding and migration of mesenchymal stem cells (MSCs) while the high fiber density surface would have smaller pore size to prevent MSCs from falling through while having the porosity for diffusion of nutrient and oxygen through the wall. MSCs and differentiated vascular smooth muscle cells (dVSMC) seeded on the patch were found to adhere well and proliferate in the scaffold.
Published date: 14 March 2023
Last updated: 02 January 2024
▼ Reference
-
Gao Y, Yi T, Shinoka T, Lee Y U, Reneker D H, Breuer C K, Becker M L. Pilot Mouse Study of 1 mm Inner Diameter (ID) Vascular Graft Using Electrospun Poly(ester urea) Nanofibers. Advanced Healthcare Materials 2016. Article in press
-
He W, Ma Z, Teo W E, Dong Y X, Robless P A, Lim T C, Ramakrishna S. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. J Biomed Mater Res A 2009; 90: 205.
-
Liu Y, Xiang K, Chen H, Li Y, Hu Q. Composite vascular repair grafts via micro-imprinting and electrospinning. AIP Advances 2015; 5: 041318.
Open Access
-
Matsushita H, Inoue T, Abdollahi S, Yeung E, Ong C S, Lui C, Pitaktong I, Nelson K, Johnson J, Hibino N. Corrugated nanofiber tissue-engineered vascular graft to prevent kinking for arteriovenous shunts in an ovine model. JVS: Vascular Science 2020; 1: 100.
Open Access
-
Mayoral I, Bevilacqua E, Gómez G, Hmadcha A, González-Loscertales I, Reina E, Sotelo J, Domínguez A, Pérez-Alcántara P, Smani Y, González-Puertas P, Mendez A, Uribe S, Smani T, Ordoñez A, Valverde I. Tissue engineered in-vitro vascular patch fabrication using hybrid 3D printing and electrospinning. Materials Today Bio 2022; 14: 100252.
Open Access
- Meng X, Wang X, Jiang Y, Zhang B, Li K, Li Q. Suture retention strength of P(LLA-CL) tissue-engineered vascular grafts. RSC Adv. 2019; 9: 21258.
Open Access
-
Tillman B W, Yazdani S K, Lee S J, Geary R L, Atala A, Yoo J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009; 30: 583
-
Uttayarat P, Perets A, Li M, Pimton P, Stachelek S J, Alferiev I, Composto R J, Levy R J, Lelkes P I. Micropatterning of three-dimensional electrospun polyurethane vascular grafts. Acta Biomaterialia 2010; 6: 4229.
-
Wu C, Wang H, Cao J. Tween-80 improves single/coaxial electrospinning of three-layered bioartificial blood vessel. J Mater Sci: Mater Med 2023; 34: 6.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech