Electrospun nanofibers with its high specific surface area are very attractive for applications where performance is related to surface area. Further, in fibrous form, the catalytic material can be self-supporting unlike nanoparticles which have high surface area individually but when they aggregate, the specific surface area drops. Although nanofibers with catalytic properties have shown good performance, there are still ways to further improve it. This may come about by further increasing the porosity of the fiber, introducing confinement effect by means of hollow fibers or adding a second material to complement the catalytic function.
Increase fiber porosity
Where there are more surface area for reaction, catalytic rate will generally increase as a result of that. Tests by Li et al (2012) showed that smaller grain size nanofibers exhibits greater specific surface area and better self-photosensitization of Rhodamine (RhB). Since increasing the surface area of the nanofibers would enhance the performance of nanofibers this has led to several researches into introducing pores to the nanofibers. Liu et al (2012) mixed carbon nanosphere into TiO2 precursor solution before electrospinning. The resultant nanofibers which contained the nanospheres undergo calcination to form TiO2 nanofibers and during this process, the nanospheres were burnt off, leaving behind porous TiO2 nanofibers. Comparing the photocatalytic performance of porous TiO2 nanofibers, solid TiO2 nanofibers and TiO2 nanoparticles, porous TiO2 works the best but solid nanofibers showed the poorest result. Therefore, the better performance can be attributed to the high surface area of porous TiO2.
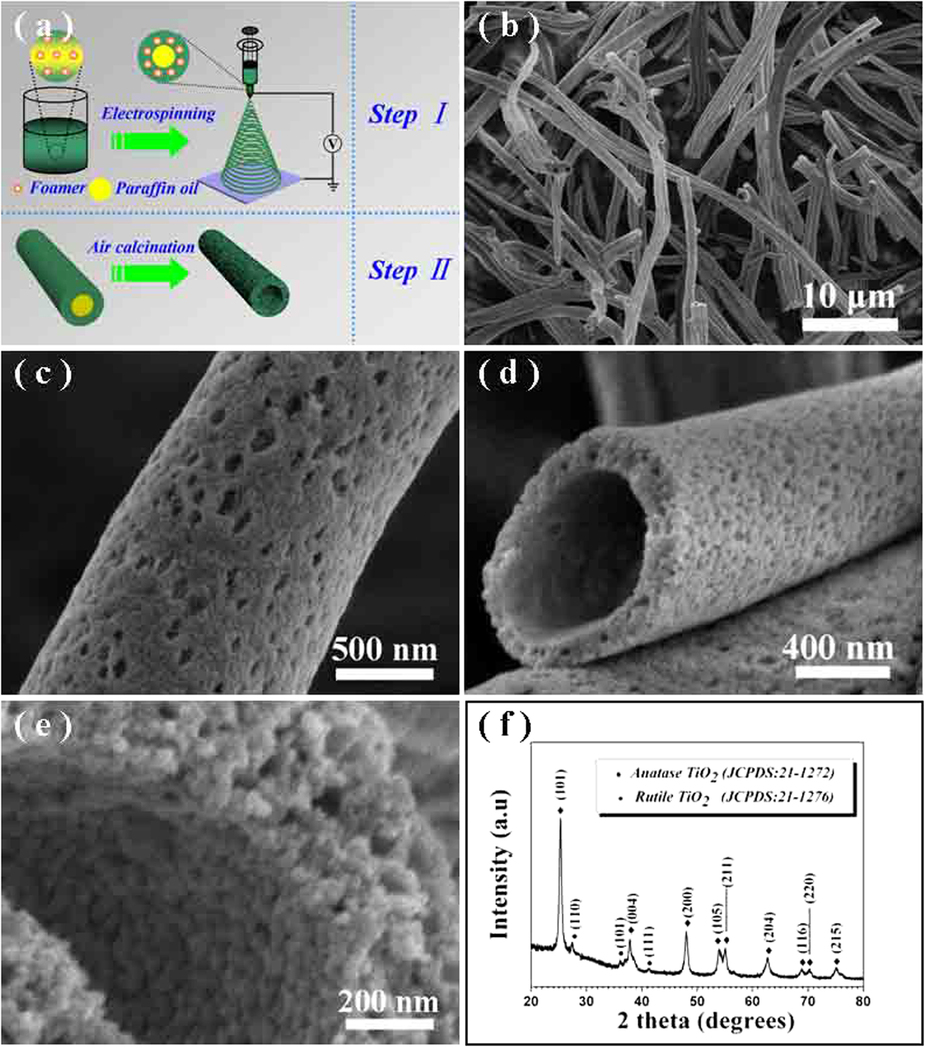
Foaming agents may be added to facilitate the formation of mesoporous fibers. Foaming agents added to the solution will decompose into vapor phases during electrospinning and the escaping vapors will create homogeneous and thoroughly mesoporous fibers. Hou et al (2015) used foaming agent, diisopropyl azodiformate (DIPA) to mix into a solution of polyvinylpyrrolidone (PVP) and butyl titanate (TBOT). CTAB and paraffin oil was later added to the solution for the formation of titanium dioxide (TiO2) mesoporous tubes. Following sintering, the mesoporous TiO2 tubes were tested for its photocatalytic activity for the evolution of hydrogen. The mesoporous TiO2 tubes were able to exhibit significantly higher photocatalytic activity at a rate of 499 µmol g-1.h-1 compared to P25 nanoparticles which was at 198 µmol g-1.h-1. The photocatalytic stability in the mesoporous TiO2 tubes was also greater than P25 nanoparticles with no noticeable drop in its activity after 3 cycles of recovery and re-use.
Hollow Fibers
Electrospun nanofibers may be made into hollow nanofibers using several means such as using the nanofibers as a template or through self-organization of the ions such that hollow tubes are formed after sintering. Hollow fibers will have a higher specific surface area compared to solid nanofibers. Better catalytic performance of hollow fibers may also be attributed to the confinement of the reacting substances within the lumen. Chen et al (2017) tested the catalytic efficiency of electrospun solid, hollow and fiber in tube antimony-doped tin oxide nanofibers (ATONF) with electrodeposited Pt nanoparticles. Solid, hollow and fiber in tube electrospun ATONFs were formed by controlling the heating rate during calcination of its precursors. Following electrodeposition of Pt nanoparticles, it was found that for hollow tube ATONFs, the Pt nanoparticles were located in the hollow channels of the nanofibers instead of on the exposed surface. While this phenomenon requires further investigation, it may be due to lower nucleation energy on the concave inner wall of the tube. Catalytic activity on oxygen reduction reaction (ORR) was the highest on the Pt nanoparticles coated hollow ATONFs compared to Pt nanoparticles coated solid ATONFs and conventional antimony-doped tin oxide powders. Higher ORR in hollow ATONF versus solid ATONF despite having the same Pt loading and nanoparticles size may be attributed to enhanced probability of O2 collision onto the catalytic Pt surface within the confines of the nanotube.
Composite fibers
Additives may be added to the electrospun nanofibers to improve the catalytic performance of the material. Dai et al (2010) fabricated a layered composite inorganic fibrous membrane containing Pt nanoparticles. First, TiO2 nanofibers were formed by sintering its electrospun precursor fibers. This is then coated with polyvinyl pyrrolidone (PVP) stabilised Pt nanoparticles followed by another layer of SiO2 nanoparticles. The presence of SiO2 nanoparticles was found to stabilise the Pt nanoparticles and prevent their aggregation during sintering at temperatures above 350 °C. Catalytic activity of the composite was determined using methyl red for the model reaction. It was shown that even with the SiO2 sheath over the Pt nanoparticles layer, methyl red molecules were able to penetrate through the porous sheath layer for catalytic reaction.
To enhance photocatalytic activity of inorganic material in visible light, dopants may be added to reduce the energy band gap. Samadi et al (2012) doped electrospun ZnO nanofibers with multi-walled carbon nanotube (MWCNT) and the doped ZnO demonstrated photocatalytic activity in visible light while pure ZnO nanofibers were inactive. Other techniques such as nitridation of TiO2 have also been used successfully to enhance photocatalytic performance in visible light [Li et al 2012b]. Ag is another element known to enhance the photocatalytic ability of TiO2. Mishra et al (2012) fabricated electrospun TiO2 nanofibers doped with Ag nanoparticles. TiO2 nanofibers were produced by annealing of titania precursor sol. To incorporate Ag nanoparticles, Ag-EtOH solution were added to the titania precursor solution containing polyvinyl pyrrolidone (PVP). Comparing the catalytic performance between pure TiO2 nanofibers and TiO2/Ag nanoparticles nanofibers, the latter showed 10.3 times higher than that by pure TiO2 nanofibers. The enhanced photocatalytic performance was attributed to electron-trapping by Ag nanoparticles to dissociate photogenerated electron-hole pairs. Lu et al (2019) demonstrated the enhancement in the photocatalytic performance of ZnO nanorod projections on polyimide/AG (PI/Ag) fibers when Ag nanoparticles were coupled with the ZnO nanorods. When Ag were embedded in the PI fiber matrix, the photocatalytic degradation rate of methylene blue (MB) was 87% with the ZnO projections. However, when Ag nanoparticles were coupled the ZnO projections on the same PI/Ag nanofiber base, the photocatalytic degradation rate increased to 98%. The synergistic effect of Ag on ZnO has been attributed to the Ag nanoparticles functioning as an electron sink that reduce the recombination of photoinduced electrons and holes which is the same as the effect on electrospun TiO2 nanofibers doped with Ag nanoparticles reported by Mishra et al (2012).
Duan et al (2019) constructed a composite of TiO2 loaded with Au nanoparticles by electrospinning for photodegradation of rhodamine B (RB). The addition of Au nanoparticles increases the photodegradation of rhodamine B (RB) under UV irradiation, visible and natural lights. The most significant improvement is seen in the degradation of RB in visible and natural lights Under natural light, degradations of RB by using TiO2/Au nanofibers as photocatalysts reached almost 100% in 270?min irradiation while the degradation by using pure TiO2 nanofibers was just 40%. The superior performance of TiO2/Au may be attributed to the plasmonic resonance absorption of Au nanoparticles and effective separation of photogenerated electrons and holes by the TiO2/Au heterojunction structures. Bairamis et al (2019) doped TiO2 with g-C3N4 to improve its photocatalytic performance on methylene blue under simulated sunlight irradiation. Increasing g-C3N4 in TiO2 increases the degradation of methylene blue. This has been attributed to interfacial transfer of carriers and the corresponding generation of -OH radicals due to the presence of g-C3N4. A Z-scheme mechanism was suggested for the generation of -OH radicals by the composite. Photogenerated holes were maintained at the VB of TiO2 while the excited electrons in the CB of TiO2 combined with the holes in the VB of g-C3N4. Excited electrons on the CB of g-C3N4 are scavenged by oxygen while the generated holes at the VB of TiO2 generated -OH radicals. The trapping of electrons at the CB of TiO2 by the holes of g-C3N4 reduces recombination and this resulted in higher photocatalytic efficiency.
Taking advantage of the improved function of hollow fibers, Guo et al (2021) constructed electrospun NaYF4/Yb/Tm/TiO2 hollow composite fibers for photocatalytic application. The hollow composite fibers were formed using a co-axial electrospinning setup. The inner core was filled with cyclohexane and paraffin which will be removed during the calcination process to form the hollow core. NaYF4/Yb/Tm/TiO2 nanoparticles were added to the cyclohexane and paraffin core during electrospinning. The shell was formed by TiO2/PVP precursor solution, Following calcination, NaYF4/Yb/Tm nanoparticles were observed to line the inner wall of the hollow electrospun fibers. For calcinated pure TiO2 hollow fibers, the inner wall was smooth. Photocatalytic performance of the fibers were tested on rhodamine B (RB). The maximum absorption peak was found to be at light wavelength of 550 nm. After 2 h of reaction, the degradation efficiency of the hollow TiO2 fibers containing NaYF4/Yb/Tm nanoparticles was at 99.12% which is significantly better than hollow pure TiO2 fibers with a degradation efficiency of 83.79%.
Maafa et al (2021) constructed a core-shell inorganic nanofiber by electrospinning a precursor solution of poly(vinyl alcohol), zinc acetate dihydrate, and cadmium acetate dihydrate followed by calcination. The resultant inorganic nanofiber was found to exhibit a Cd-rich core and Zn-rich shell. Such composite nanofiber has a much higher photocatalytic than pure ZnO nanofiber with methylene blue (MB) photo-degradation at 98% and 42% under 210 min of sunlight Irradiation for CdO/ZnO core/shell nanofibers and ZnO nanofibers, respectively. The better photocatalytic performance of CdO/Zn nanofibers over ZnO nanofibers may be attributed to lower band gap of CdO/Zn. With CdO/ZnO core/shell nanofibers, the generated electrons from irradiation of the surface ZnO were transferred to the conduction band of the CdO core. This reduces the recombination rate of the generated electron-hole pairs. The photo-generated holes and electrons would facilitate the formation of hydroxyl free radicals from water which oxidizes and degrades organic pollutants.
Photocatalytic Material Location and Distribution
The location of the photocatalytic material on the electrospun membrane may have a significant influence on its photocatalytic activity. AlAbduljabbar et al (2021) tested the efficacy of degrading methyl orange with TiO2 nanoparticles either adhered to the surface of electrospun polyacrylonitrile (PAN) fibers or blended within the PAN fibers. The electrospun PAN membrane with surface coated TiO2 nanoparticles was constructed by electrospraying a suspension of TiO2 nanoparticles over the prefabricated PAN nanofibers membrane. Comparing their photocatalytic activity, it was shown that PAN nanofibers membrane with surface coated TiO2 nanoparticles has a much higher activity at 92% against the TiO2/PAN blended sample at 49.6%. The difference in photocatalytic activity can be attributed to the greater exposure of surface TiO2 nanoparticles to methyl orange. Having TiO2 nanoparticles on the surface also increases the surface roughness and contributes to increased surface area of the membrane.
For electrospun fibers to be an effective carrier for catalytic particles, the nanoparticles have to be uniformly dispersed throughout the matrix. To construct nanofibers for uniform dispersion of negatively charged platinum nanoparticles, Li et al (2017) electrospun amino-functionalized polyacrylonitrile (PAN) nanofibers by electrospinning a PAN and 3-aminopropyltriethoxysilane (APS) mixed precursor solution. The electrospun fibers were dipped into platinum sol followed by reduction to form platinum nanoparticles (PtNPs). The positive-charged PAN/APS nanofibers were able to bind with the negative-charged platinum nanoparticles (PtNPs) through electrostatic interaction. A high density of PtNPs was found to be uniformly distributed on the surface of the electrospun nanofibers.
Placement of photocatalytic material
Electrospun fibers are commonly used as carriers for photocatalytic nanoparticles. As a carrier, factors such as the location of the photocatalytic material and the bonding between the photocatalytic material and carrier may have a strong influence on the photocatalytic activity. AlAbduljabbar et al (2021) used electrospun chitosan nanofibers as the carrier for TiO2 nanoparticles. Two methods of loading TiO2 nanoparticles were being investigated. The first method involves surface functionalization of chitosan nanofibers followed by electrospraying to deposit TiO2 nanoparticles on the surface of the nanofibers. In the second method, TiO2 nanoparticles were mixed into a chitosan solution and electrospun into fibers. Comparison of their photocatalytic performance was determined by the degradation of methylene blue dye. With the TiO2 nanoparticles on the surface of functionalized nanofibers, the photodegradation rate under UV light was 89.30% and a reaction rate constant k at 0.0088 min-1. For TiO2 nanoparticles that were blended into the chitosan nanofibers, the degradation efficiency under UV was only 40.26% and the reaction rate constant k was 0.0016 min-1. This significant difference in performance may be attributed to TiO2 nanoparticles on the surface of the fibers being more exposed to the UV light and in direct contact with the methylene blue molecules as compared to the blended system where the TiO2 nanoparticles were embedded within the carrier matrix material. The band gap of the TiO2 nanoparticles on the surface of functionalized nanofibers was also found to be smaller than the TiO2 nanoparticles/chitosan blended fibers. Surface roughness of chitosan nanofiber with TiO2 nanoparticles on its surface is also higher and this increases the exposed reaction site with the dye molecules.
Environmental condition
The environmental condition where the photodegradation takes place has a significant influence on its rate. Higher reaction temperature generally increases the photodegradation rate. This is due to faster electron-hole formation and faster movement of the dye molecules. However, an increase in temperature beyond 80 °C may be detrimental as the electron-hole recombination rate increases [Maafa et al 2022].
Another factor is the light intensity on the photocatalyst. An increase in light intensity increases the photons striking the photocatalyst and generating more electron-hole pairs. Maafa et al 2022 showed that with their electrospun derived carbon /NiTiO3/ TiO2 nanofibers membrane, the photodegradation of Methylene blue (MB) increases with increasing light intensity.
Published date: 29 August 2017
Last updated: 22 August 2023
▼ Reference
-
AlAbduljabbar FA, Haider S, Ali FAA, Alghyamah AA, Almasry WA, Patel R, Mujtaba IM. Efficient Photocatalytic Degradation of Organic Pollutant in Wastewater by Electrospun Functionally Modified Polyacrylonitrile Nanofibers Membrane Anchoring TiO2 Nanostructured. Membranes. 2021; 11(10):785.
Open Access
-
AlAbduljabbar F A, Haider S, Ali F A A, Alghyamah A A, Almasry W A, Patel R, Mujtaba I M. TiO2 nanostructured coated functionally modified and composite electrospun chitosan nanofibers membrane for efficient photocatalytic degradation of organic pollutant in wastewater, Journal of Materials Research and Technology 2021; 15: 5197.
Open Access
-
Bairamis F, Konstantinou I, Petrakis D, Vaimakis T. Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue. Catalysts 2019; 9(11): 880.
Open Access
-
Chen S, Du H, Wei Y, Peng L, Li Y, Gan L, Kang F. Fine-tuning the Cross-Sectional Architecture of Antimonydoped Tin Oxide Nanofibers as Pt Catalyst Support for Enhanced Oxygen Reduction Activity. Int. J. Electrochem. Sci. 2017; 12: 6221.
Open Access
-
Dai Y, Lim B, Yang Y, Cobley C M, Li W, Cho E C, Grayson B, Fanson P T, Campbell C T, Sun Y, Xia Y. A Sinter-Resistant Catalytic System Based on Platinum Nanoparticles Supported on TiO2 Nanofibers and Covered by Porous Silica. Angew Chem Int Ed 2010; 49: 8165.
-
Duan Z, Huang Y, Zhang D, Chen S. Electrospinning Fabricating Au/TiO2 Network-like Nanofibers as Visible Light Activated Photocatalyst. Scientific Reports 2019; 9: 8008.
Open Access
-
Guo F, Guo Z, Gao L, Qi D, Yue G, Wang N, Zhao Y, Li N, Xiong J. Electrospun Core-Shell Hollow Structure Cocatalysts for Enhanced Photocatalytic Activity. Journal of Nanomaterials 2021; 2021: 9980810.
Open Access
-
Hou H, Shang M, Wang L, Li W, Tang B, Yang W. Efficient Photocatalytic Activities of TiO2 Hollow Fibers with Mixed Phases and Mesoporous Walls. Scientific Reports 2015; 5: 15228.
Open Access
-
Li J, Qiao H, Du Y, Chen C, Li X, Cui J, Kumar D, Wei Q. Electrospinning Synthesis and Photocatalytic Activity of Mesoporous TiO2 Nanofibers. The Scientific World Journal 2012; 2012: 154939.
Open Access
-
Li H, Zhang W, Huang S, Pan W. Enhanced visible-light-driven photocatalysis of surface nitrided electrospun TiO2 nanofibers. Nanoscale 2012b; 4: 801.
-
Li P, Zhang M, Liu X, Su Z, Wei G. Electrostatic Assembly of Platinum Nanoparticles along Electrospun Polymeric Nanofibers for High Performance Electrochemical Sensors.
Nanomaterials 2017; 7: 236.
-
Liu S, Liu B, Nakata K, Ochiai T, Murakami T, Fujishima A. Electrospinning Preparation and Photocatalytic Activity of Porous TiO2 Nanofibers. Journal of Nanomaterials 2012; 2012; 491927.
Open Access
-
Lu F, Wang J, Chang Z, Zeng J. Uniform deposition of Ag nanoparticles on ZnO nanorod arrays grown on polyimide/Ag nanofibers by electrospinning, hydrothermal, and photoreduction processes. Materials & Design 2019; 181: 108069
Open Access
-
Maafa IM, Al-Enizi AM, Abutaleb A, Zouli N I, Ubaidullah M, Shaikh SF, Al-Abdrabalnabi MA, Yousef A. One-pot preparation of CdO/ZnO core/shell nanofibers: An efficient photocatalyst. Alexandria Engineering 2021; 60: 1819.
Open Access
-
Maafa IM, Ali MA. Enhanced Organic Pollutant Removal Efficiency of Electrospun NiTiO3/TiO2-Decorated Carbon Nanofibers. Polymers. 2023; 15(1):109.
Open Access
-
Mishra S, Ahrenkiel S P. Synthesis and Characterization of Electrospun Nanocomposite TiO2 Nanofibers with Ag Nanoparticles for Photocatalysis Applications. Journal of Nanomaterials 2012; 2012: 902491.
Open Access
-
Samadi M, Shivaee H A, Zanetti M, Pourjavadi A, Moshfegh A. Visible light photocatalytic activity of novel MWCNT-doped ZnO electrospun nanofibers. Journal of Molecular Catalysis A: Chemical 2012; 2012: 359.
▲ Close list
 ElectrospinTech
ElectrospinTech