Electrospun membrane is known to have high porosity but small pore size which limits cell penetration. This is often seen as a limitation to its application in tissue regeneration where larger pore size for cell infiltration is preferred. However, this property is an advantage for certain biomedical applications where it function as a barrier against cell and microbes penetration while its high porosity allows transfer of fluid, smaller biomolecules and gas.
Typical pore size of electrospun fibrous membrane is less than 5µ and this is able to prevent bacteria from reaching the wound. To demonstrate the effectiveness of electrospun layer in filtering out microbes and bacteria, Lev et al (2012) first electrospun a layer of polyurethane over a polypropylene supporting substrate. With a electrospun nanofibers weight of 3.8 g/m2, it is able to filter out E. coli although at weight less than this, E. coli was able to get pass the membrane. Chaudhary et al (2014) used an electrospun polyacrylonitrile-silver composite filter media to cover a nutrient media in room condition and passes ambient air through the filter media. When compared to the negative control which is without the protective filter media, the nutrient media protected by the nanofibrous filter remains free of bacteria growth after two months while the unprotected nutrient media show microorganism growth.
Due to its effectiveness as a cell barrier, electrospun fibers membrane has been tested as an anti-adhesion barrier film between injured site and the surrounding tissue. This is important for preventing peritendinous adhesion where the injured tendon repair site suffers from fibrotic adhesion which limits the mobility of the joint. Using an electrospun hyaluronic acid-grafted poly(caprolactone) (PCL-g-HA) nanofibrous membrane, Chen et al (2014) showed that PCL-g-HA electrospun membrane is effective in reducing peritendinous adhesion in an animal model. The study also showed that the presence of HA is necessary to reduce fibroblast attachment while the membrane provides a physical barrier for fibrotic adhesion. Jin et al (2022) constructed a composite pericardial functional barrier membrane with electrospun poly(l-lactide-co-caprolactone) (PLCL) nanofibers as the base material. In their modified electrospinning setup, the PLCL solution was electrospun directly into a reservoir of ethanol/water with poly(vinyl alcohol) (PVA) and melatonin dissolved in it. The low surface tension of ethanol/water meant that the deposited fibers will sink below the surface upon impact. The pores of the resultant membrane will thus be filled with PVA and melatonin. Repeated freeze-drying of the membrane causes the PVA to form macroporous and hydrophilic aerogel. Melatonin is a hormone that shows anti-inflammatory and anti-fibrosis effects. The combination of PLCL fibrous membrane, PVA aerogel and melatonin (PPMT) was shown to inhibit fibroblast and monocyte proliferation in vitro. In vivo study using a rabbit pericardial reconstruction model showed that no pericardial adhesion occurred in rabbits in the PPMT group in contrast to different degrees of postoperative adhesions in the control groups.
Electrospun membrane may also be used to construct an implantable pocket for harbouring cells that produces specific biomolecules while shielding them from host cells. High porosity and small size of the biomolecules allow them to diffuse from the pocket to the surrounding site. Krishnan et al (2011) constructed a dual membrane pocket for cellular immunoisolation for pancreatic islet transplantation. The inner membrane is a thin layer comprising of nylon fibers and has an average pore size of 0.3 µm and fiber diameter of 127 nm to prevent cell infiltration. The outer layer has a larger pore size of 1.66 µm to facilitate tissue integration. In vivo study showed that the inner membrane forms an effective barrier against cell infiltration while the outer membrane allows cell integration. In a separate study, Sojoodi et al (2013) showed that the combination of laminin coating and nanofiber surface (but not laminin coating on smooth surface and nanofiber alone) was necessary to induce significant insulin secretion from adult β cells islets. The relative ease of loading drugs into electrospun fibers may also be utilized to release drugs within the niche. Ji et al (2021) constructed an avascular niche made of axitinib-loaded PCL/collagen nanofibrous membrane for implantation into subcutaneous environments in order to maintain cartilaginous phenotype of mesenchymal stromal cells (MSCs). To construct the niche with MSCs, the electrospun membrane was first prepared. MSCs were seeded onto a nonwoven poly (lactic-co-glycolic acid) (PLGA) fabric and encapsulated within the electrospun PCL/collagen nanofibrous membrane. Tissue adhesive Histoacryl was used to seal the nanofibrous membrane pocket containing the seeded woven PLGA fabric. The loading and release of axitinib in the niche is vital to inhibit angiogenic activity since vascular invasion is a prerequisite for endochondral ossification. In vivo study of the niche seeded with MSCs implanted subcutaneously into nude mice showed that samples maintained their cartilage features even after 24 weeks of subcutaneous implantation in the 3% Axitinib and 6%.
Kasoju et al (2018) used a combination of 3D printed framework to support electrospun fiber collection and sacrificial salt pellets to create spacings between the framework and the electrospun layers at both sides of the framework. Polyamide 66 (PA66) was selected as the material to be electrospun. The framework incorporated an injection port where the cells can be loaded into the pocket between the electrospun layers. Using fibroblasts and lymphocytes as model cell types, the device was able to contain fibroblasts while keeping the immune cells outside. The capsule was also able to allow free diffusion of model biomolecules (insulin, albumin and Ig G).
In cartilage and bone repair, the interface between the two needs to be considered. Not only are they inhibited by different cells, their functions are also different hence there is a need to direct regeneration in separate directions if a single scaffold is to be used. Mellor et al (2020) used multi-phasic 3D-bioplotting to construct the main body of the scaffold for cartilage and bone repair. In the part meant for cartilage regeneration, decellularized bovine cartilage extracellular matrix (dECM) hydrogel was injected to the 3D-bioplotted poly(ε-caprolactone) (PCL) scaffold. For bone regeneration, 20% β-tricalcium phosphate (TCP)/ 80% PCL was 3D-bioplotted. An electrospun PCL membrane layer was added between the two 3D-bioplotted scaffolds. This electrospun PCL membrane layer is needed to inhibit cell migration between the scaffolds layers. In a clinical setting, this layer will also prevent blood vessels from invading the chondrogenic portion of the scaffold. In vitro study using human adipose-derived stem cells (hASC) showed that the electrospun membrane was able to effectively separate the cell populations while evidence of site-specific hASC osteogenesis and chondrogenesis was observed.
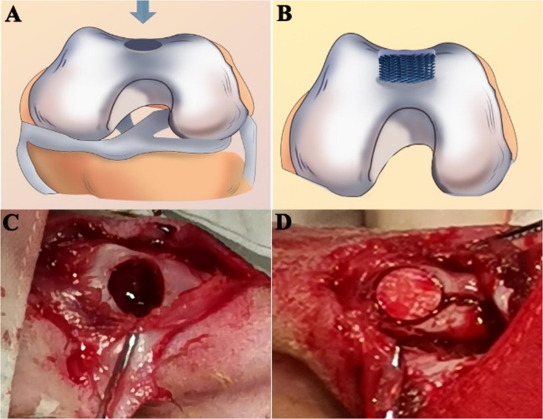
Electrospun membrane has also been used as a barrier to prevent migration of fibrous connective tissues into the scaffold intended for bone regeneration. Liu et al (2021) used electrospinning and 3D printing to construct a hybrid bi-layer scaffold for this purpose. Polycaprolactone/gelatine (PCL/Gel) nanofibre membranes was first electrospun followed by conjugation with heparin. 3D printing of PCL/Gel/nano-hydroxyapatite (n-HA) scaffold was carried out over the PCL/Gel-heparin membrane to form a bi-layer scaffold. <>In vivo study on a rabbit osteochondral defect model showed that the hybrid bi-layer scaffold group showed a higher degree of new bone formation than the 3D printed PCL/Gel/n-HA scaffold and control group after 5 and 20 weeks. With the electrospun membrane positioned on the surface and the defect filled in by the 3D printed bulk volume, the membrane prevents surrounding fibrous connective tissues from migrating into the bone defect. The inner layer with the 3D printed macropores allows bone regeneration while supporting the surface membrane.
Tian et al (2023) seek to mimic the ECM structure of gastrointestinal mucosa with ridge-like surface texture and differentiated layers of cells where there is a fibroblast populated interior layer of mucosa and epithelial cells on the inner rough surface. A copper collector cooled to -20 °C was used to induce the formation of ridge-like structure as the electrospun polycaprolactone (PCL) fibers deposited on it. With this setup, the resultant membrane has a smooth underside which was in contact with the collector and a rough surface with the ridge-like structures. In a customized chamber, fibroblasts were cultured on the smooth surface of the membrane and epithelial cells on the rough side. The chamber prevented the cells from one side of the membrane to cross to the other side from the edges of the membrane. The pores between the fibers were too small for the cells to pass through the membrane but sufficiently large to allow biomolecules to pass. The EGF secreted by the fibroblasts was able to pass through the membrane and stimulate the epithelial cells on the rough side, demonstrated by the secretion of Mcl-1 and c-Myc RNA by epithelial cells.
Published date: 07 June 2016
Last updated: 16 January 2024
▼ Reference
-
Chaudhary A, Gupta A, Mathur R B, Dhakate S R. Effective antimicrobial filter from electrospun polyacrylonitrile-silver composite nanofibers membrane for conducive environment. Adv. Mat. Lett. 2014; 5: 562.
-
Chen S H, Chen C H, Shalumon K T, Chen J P. Preparation and characterization of antiadhesion barrier film from hyaluronic acid-grafted electrospun poly(caprolactone) nanofibrous membranes for prevention of flexor tendon postoperative peritendinous adhesion. International Journal of Nanomedicine 2014; 9: 4079.
Open Access
-
Ji TJ, Feng B, Shen J, Zhang M, Hu YQ, Jiang AX, Zhu DQ, Chen YW, Ji W, Zhang Z, Zhang H, Li F. An Avascular Niche Created by Axitinib-Loaded PCL/Collagen Nanofibrous Membrane Stabilized Subcutaneous Chondrogenesis of Mesenchymal Stromal Cells. Adv. Sci. 2021; 8: 2100351.
Open Access
-
Jin D, Yang S, Wu S, Yin M, Kuang H. A functional PVA aerogel-based membrane obtaining sutureability through modified electrospinning technology and achieving promising anti-adhesion effect after cardiac surgery. Bioactive Materials 2022; 10; 355.
Open Access
-
Kasoju N, George J, Ye H, Cui Z. Sacrificial Core-Based Electrospinning: A Facile and Versatile Approach to Fabricate Devices for Potential Cell and Tissue Encapsulation Applications. Nanomaterials 2018; 8(10): 863.
Open Access
-
Krishnan L, Clayton L R, Boland E D, Reed R M, Hoying J B, Williams S K. Transplant Proc. 2011; 43: 3256.
Open Access
-
Lev J, Holba M, Kalhotka L, Mikula P, Kimmer D. Improvements in the Structure of Electrospun Polyurethane Nanofibrous Materials Used for Bacterial Removal from Wastewater. International Journal of Theoretical and Applied Nanotechnology 2012; 1: 16. Open Access
-
Liu J, Zou Q, Wang C, Lin M, Li Y, Zhang R, Li Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Materials & Design 2021; 210: 110047
Open Access
-
Mellor L F, Nordberg R C, Huebner P, Mohiti-Asli M, Taylor M A, Efird W, Oxford J T, Spang J T, Shirwaiker R A, Loboa E G. Investigation of multiphasic 3D-bioplotted scaffolds for site-specific chondrogenic and osteogenic differentiation of human adipose-derived stem cells for osteochondral tissue engineering applications. J Biomed Mater Res. 2020;108B:2017.
Open Access
-
Tian W, Liu X, Ren K, Fuh J Y H, Zhang X, Bai T, Wu B. Biomimetic Janus film fabricated via cryogenic electrospinning for gastrointestinal mucosa repair. Materials & Design 2023; 228: 111839.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech