Cell seeding on a two dimensional electrospun membrane is very straight forward and is the same as seeding cells on tissue culture plates. The cells suspended in the media will just settle on the membrane under its weight and adhere to the surface of the membrane. Several studies have shown electrospun fibres membrane facilitates rapid cell adhesion. However, many applications of electrospun scaffolds require the cells to be found within the depth of the membrane or the scaffold. Alternative seeding methods will be needed to incorporate cells beyond the surface of the scaffold.
Filling a highly porous three dimensional electrospun fibrous block with cell suspension will result in most cells settling to the bottom of the well instead of attaching on the fibres. This is understandable since the rounded cells would easily fall through large pore openings. Such difficulties in cell seeding are not restricted to electrospun 3D scaffold. Other highly open 3D scaffold also face the same challenge. Electrospinning has been used for encapsulation of cells within the nanofibers [Townsend-Nicholoson et al 2006, Ang et al 2014]. In scaffold constructed using fuse deposition, electrospun fibers have been used in combination with it to create an environment that resembles extracellular matrix (ECM). However, having the electrospun mesh layers do not address the challenge of seeding the cells. When the electrospinning duration is too long, the pore size between the interconnecting fibers will be too small for cell infiltration. Too short a spinning duration will result in insufficient fiber covering the pores between the struts resulting in poor cell distribution throughout the scaffold. Lee and Kim (2014) used 10s electrospinning duration to deposit fibers between strut layers. To transfer cells within the scaffold, struts comprising of alginate and poly(ethylene oxide) with cells loaded within were laid between PCL struts. After 7 days of culture, the cells were found to be well distributed throughout the scaffold.
Erben et al (2022), both melt blowing and electrospinning uses multiple spinnerets to produce a 3D poly-ε-caprolactone (PCL) fibers scaffold. In their setup, the electrospinning spinnerets were pointed towards the flight path of the melt blown fibers such that the melt blown fibers caught the electrospun fibers and both fibers were collected using a rotating drum collector. SEM images showed the scaffold with thickness of 6 mm was made of randomly oriented microfibers and nanofibers from melt-blowing and electrospinning respectively. Their study showed that the best cell seeding method is to use bioink hydrogel loaded with cells and a needle to inject the hydrogel and cells into the center of the scaffold. The seeded human bone osteoblasts-MG63 showed greater proliferation and confluent growth compared to seeding cell suspension on the top surface of the scaffold.
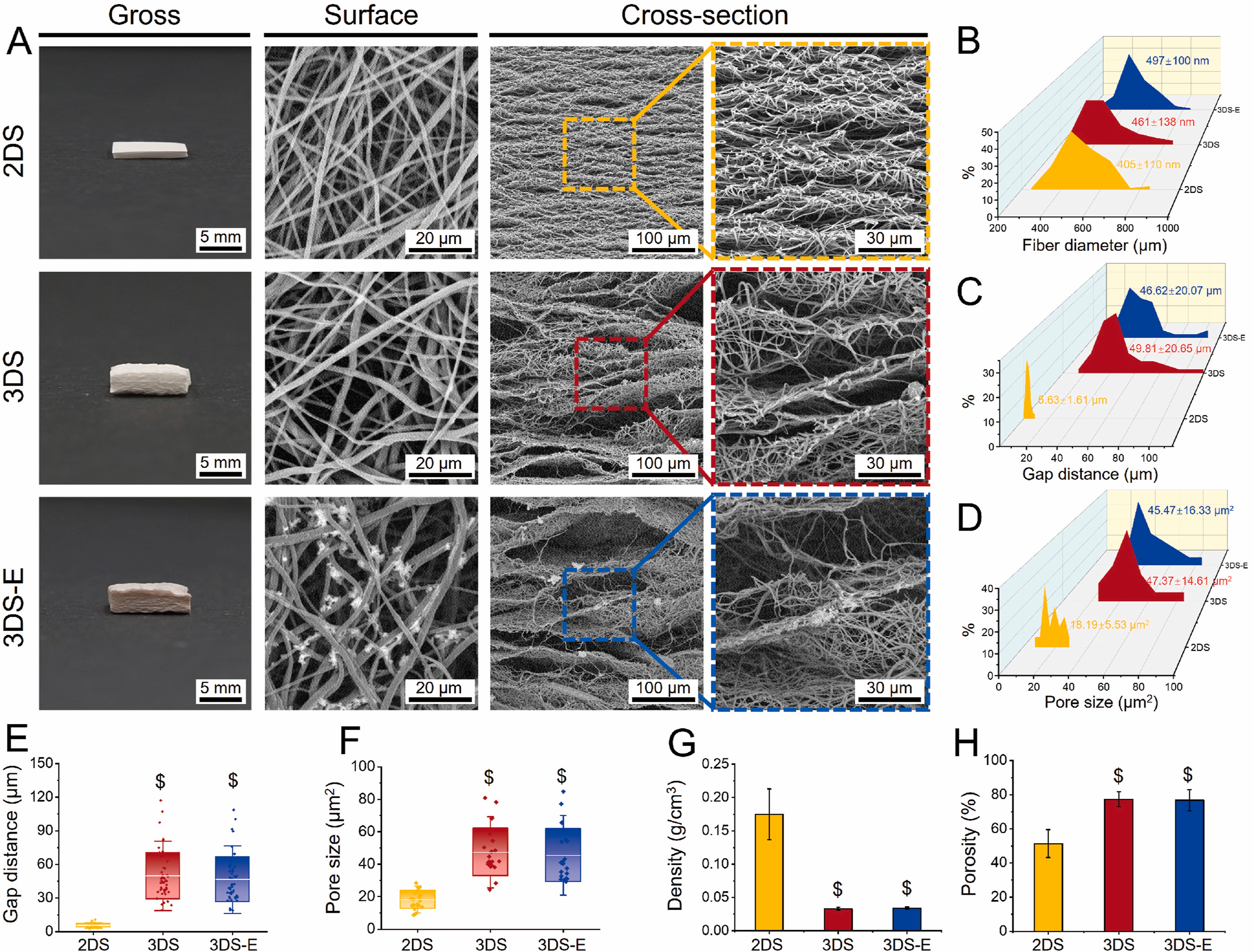
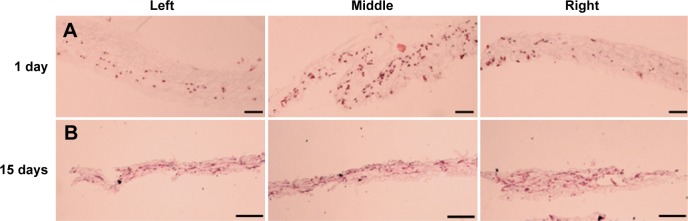
Depending on the method of fabricating the electrospun 3D scaffold, it is possible to construct expanded layered 3D scaffolds. This is usually created using porogen or other gas foaming techniques to expand the electrospun membrane. An advantage of this structure is that the layered nanofibers become flat surfaces for the cells to rest on. The interconnected fibers on each layer form pores that are generally small but also contain randomly distributed fibers that are further apart. Chen et al (2024) used a gas-foaming technique to expand and increase the thickness of electrospun poly(L-lactide-co-ε-caprolactone) (PLCL) / silk fibroin (SF) membrane.This was achieved by soaking pieces of PCL/SF electrospun 2D membranes (2DS) in 0.5 M NaBH4 aqueous solution at room temperature for 30 min. The resultant 3D scaffold was subsequently immersed in a solution of metal phenolic networks (MPNs) composed of epigallocatechin gallate (EGCG) and strontium ions (Sr2+) for 10 minutes followed by freeze drying to form 3D scaffold with MPNs (3DS-E). The scaffold showed distinct layers that were interconnected at random sites. In vitro studies demonstrated much better infiltration of chondrocytes in the expanded 3D electrospun scaffold (3DS) compared to the 2D electrospun scaffold (2DS). Cells in 3DS scaffolds after 4 h showed infiltration to a depth of 89 µ compared to just 20 µm for 2DS.
Seeding cells into an electrospun scaffold using cell loaded gel is generally straightforward and this may allow other substances to be incorporated into the final composite structure. Kong et al (2024) used near field melt electrospinning to construct a highly ordered and porous structure. Human adipose-derived stem cells (ADSC) were loaded in a GelMA hydrogel and cast into the constructed melt electrospun PCL nanofibers scaffold. The cells were observed to be evenly distributed throughout the scaffold. After incubation for 2 days, the composite scaffold was washed to remove the hydrogel that filled the pores. Cells can be seen adhering to the fibers after the washing process.
Depending on the specific requirements of the scaffold, the duration of electrospinning may be tailored according to whether the electrospun layer is to allow cells to infiltrate or to prevent the cells from falling through. Mayoral et al (2022) used a fused deposition method with polycaprolactone (PCL) to construct a patch with four-layered grid pattern design. To deposit electrospun fibers on the 3D printed patch, the collector was given a negative high voltage to direct the electrospinning jet to the collector while a positive high voltage was applied to the nozzle for electrospinning. For the surface of the patch that faces the adventitia, a lower density of electrospun fibers were deposited and for the surface that faces the intima, a higher density of electrospun fibers were deposited. The lower fiber density surface would have greater pore size to allow seeding and migration of mesenchymal stem cells (MSCs) while the high fiber density surface would have smaller pore size to prevent MSCs from falling through while having the porosity for diffusion of nutrient and oxygen through the wall.
In most applications of electrospun scaffold, it is desirable that the cells populate its entire depth. However, small pore size between electrospun fibres made it almost impossible for the cells to migrate into the depth of the membrane. One possible technique to incorporate cells into the depth of the electrospun membrane is simultaneous electrospinning and cell seeding. Cell seeding may be carried out by electrospraying where the cell suspension is sprayed using a high voltage similar to electrospinning. Braghirolli et al (2015) used this concept to incorporate mesenchymal stem cells (MSC) into poly(lactide-coglycolide) (PLGA) nanofibers scaffold. Histological analysis after 24 hours showed good cell distribution throughout the scaffold.
Yang et al (2022) used coaxial nozzle electrospinning to distribute cells across the depth of the scaffold. Human bone mesenchymal stem cells (hBMSCs) used in their study was loaded onto the shell of their poly (3-hydroxybutyrate-co-4-hydroxybutyrate) (PHB) core and poly (vinyl alcohol) (PVA) shell fiber. PVA was used as the shell material as it is water soluble and the cell culture media containing hBMSCs was mixed with the PVA solution before electrospinning. Although the core material, PHB, was dissolved in CH2CL2, the solvent does not seem to have a significant impact on the viability of the cells. The cell survival rate at 3 days was found to be 97% which is the same as the control where cells are seeded onto electrospun PHB/PVA fibers. Since PVA is water soluble, it may gradually solubilize in an aqueous environment thus freeing the cells to migrate or reconstruct the scaffold.
In a study by Lu et al (2023), scaffolds were prepared by co-electrospinning of human umbilical cord mesenchymal stem cells (HUCMSCs) and core-shell fibers (P(AGD)) with the shell made of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P34HB) and the core loaded with aqueous solution containing osteogenic compounds L-ascorbic acid-2-phosphate magnesium (ASP), β-glycerophosphate sodium (GP) and dexamethasone (DEX) such that the cells were evenly distributed throughout the P(AGD) scaffold (P(AGD)-CES). In vivo study by subcutaneous implantation of P(AGD)-CES scaffold in rabbit was compared with scaffolds prepared with in vitro co-culturing of HUCMSCs on P(AGD), P34HB nanofiber scaffold but in osteogenic media (P-(ADG)) and P34HB, for 2 weeks prior to implantation. After 8 weeks, the P(AGD)-CES in rabbits showed significant new bone formation and better results compared to the other scaffolds where HUCMSCs were cultured on the scaffolds prior to implantation. The better performance of P(AGD)-CES may be attributed to better distribution of cells throughout the depth of the scaffold compared to the others where the cells were mainly found on the surface.
Electrospinning need not be carried out on a solid collector. The process is versatile enough such that fibers may be deposited directly on a fluid media. Yang et al (2009) takes advantage of this concept to electrospin PCL/collagen onto cell culture media where cell seeding can be carried out immediately followed by electrospinning another layer of PCL/collagen fibers over the seeded cells. This process is repeated until sufficiently thick layers of fibers with cells were constructed.
Electrospinning and electrospraying of cells simultaneously has the advantage of even distribution of cells throughout the scaffold. However, an important aspect is the percentage of viable cells after the electrospinning and seeding process. Polymers used in the construction of tissue supporting scaffolds mostly dissolve in organic solvents to form the solution for electrospinning. However, these organic solvents are toxic to cells in varying degrees. Hexafluorisopropanol (HFIP) is a common solvent used in dissolving collagen for electrospinning and it may also be used for dissolving many other biodegradable polymers such as polylactide (PLA) and polycaprolactone (PCL). However a comparison of cell viability after simultaneous cell electrospraying and electrospinning of polylactide/collagen dissolved in HFIP and polylactide dissolved in dichloromethane or dichloromethane/methanol solvent, the cell viability is the worst with HFIP solvent with less than 50% survivinget [Schuttler et al 2020]. This can be mitigated by increasing the electrospinning distance from 6 cm to 12 cm with cell viability increases to about 70%. At a higher tip to collector distance, more solvent would have vaporized and this reduces the toxicity effect on cells. Another consideration is the type of collector for the fibers and cells. Schuttler et al (2020) showed that with a liquid collector, cell viability increases significantly compared to a conventional solid substrate collector. When a cell culture media was used in the liquid collector, the culture media has a protective effect both from dilution of any residual solvents, it also possibly prevents the cells from drying out. There is a jump in cell viability from 34% to more than 70% when a solid aluminum collector was switched to a DMEM liquid collector.
Published date: 06 September 2016
Last updated: 18 November 2025
▼ Reference
-
Ang H Y, Irvine S A, Avrahami R, Sarig U, Bronshtein T, Zussman E, Boey F Y C, Machluf M, Venkatraman. Characterization of a bioactive fiber scaffold with entrapped HUVECs in coaxial electrospun core-shell fiber. Biomatter 2014; 4: e28238.
Open Access
-
Braghirolli D I, Zamboni F, Acasigua G A X, Pranke P. Association of electrospinning with electrospraying: a strategy to produce 3D scaffolds with incorporated stem cells for use in tissue engineering. International Journal of Nanomedicine 2015; 10: 5159.
Open Access
-
Chen Y, Xu W, Pan Z, Li B, Mo X, Li Y, Wang J, Wang Y, Wei Z, Chen Y, Han Z, Lin C, Liu Y, Ye X, Yu J. Three-dimensional gas-foamed scaffolds decorated with metal phenolic networks for cartilage regeneration. Materials Today Bio, 2024; 29: 101249.
https://www.sciencedirect.com/science/article/pii/S2590006424003107 Open Access
-
Erben J, Jirkovec R, Kalous T, Klicova M, Chvojka J. The Combination of Hydrogels with 3D Fibrous Scaffolds Based on Electrospinning and Meltblown Technology. Bioengineering. 2022; 9(11):660.
Open Access
-
Kong X, Zhu D, Hu Y, Liu C, Zhang Y, Wu Y, Tan J, Luo Y, Chen J, Xu T, Zhu L. Melt electrowriting (MEW)-PCL composite Three-Dimensional exosome hydrogel scaffold for wound healing. Materials & Design 2024; 238: 112717.
Open Access
-
Lee H, Kim G H. Enhanced cellular activities of polycaprolactone/alginate-based cell-laden hierarchical scaffolds for hard tissue engineering applications. Journal of Colloid and Interface Science 2014; 430: 315.
-
Lu T, Yang L, Li Z, Liu Y, Xu S, Ye C. Immediate implantation of ultrafine fiber slow-release system based on cell electrospinning to induce osteogenesis of mesenchymal stem cells. Regen Biomater. 2023;11: rbad113.
Open Access
-
Mayoral I, Bevilacqua E, Gómez G, Hmadcha A, González-Loscertales I, Reina E, Sotelo J, Domínguez A, Pérez-Alcántara P, Smani Y, González-Puertas P, Mendez A, Uribe S, Smani T, Ordoñez A, Valverde I. Tissue engineered in-vitro vascular patch fabrication using hybrid 3D printing and electrospinning. Materials Today Bio 2022; 14: 100252.
Open Access
Schuttler K F, Bauhofer M W, Ketter V, Giese K, Eschbach D A, Yenigun M, Fuchs-Winkelmann S, Paletta J R J. Direct incorporation of mesenchymal stem cells into a Nanofiber scaffold - in vitro and in vivo analysis. Sci Rep 2020; 10: 9557.
Open Access
-
Townsend-Nicholoson A and Jayasinghe S N. Cell Electrospinning: a Unique Biotechnique for Encapsulating Living Organisms for Generating Active Biological Microthreads/Scaffolds. Biomacromolecules 2006; 7: 3364.
-
Yang L, Zhao Y, Cui D, Liu Y, Zou Q, Xu S, Luo S, Ye C. Coaxial bioelectrospinning of P34HB/PVA microfibers biomimetic scaffolds with simultaneity cell-laden for improving bone regeneration. Materials & Design 2022; 213: 110349.
Open Access
-
Yang X, Shah J D and Wang H (2009) Nanofiber Enabled Layer-by-Layer Approach Toward Three-Dimensional Tissue Formation. Tissue Engin. A 15 945-956.
▲ Close list
 ElectrospinTech
ElectrospinTech