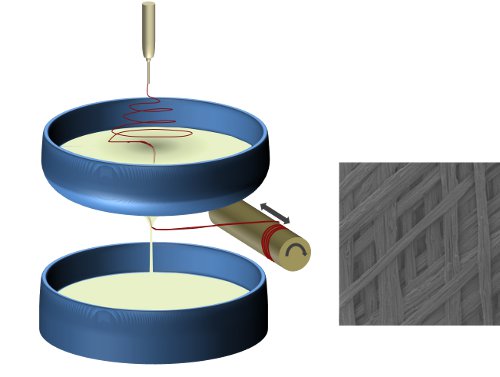
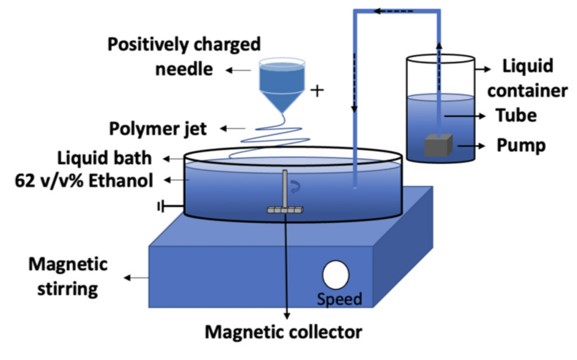
A non-solvent liquid collector provides a highly versatile alternative for collecting electrospun fibers. Such a system offers several advantages over conventional solid substrate collector although care must be taken to mitigate the risk of contact between the liquid and the charged electrode. In general, the electrospinning parameter for solid and liquid collector is the same. However, the fiber form and diameter collected may be different even if the parameters are kept the same [Kostakova et al 2014]. A liquid collector may be static or dynamic depending on the required output. Other considerations when using a non-solvent liquid collector is the surface tension of the liquid, interaction between the electrospun solution and the liquid and temperature of the liquid [Liu et al 2013]. Unlike conventional electrospinning, interaction between the spinning solution and the collection liquid needs to be considered. For some solutions, the collection liquid may aid solidification and fiber formation if the electrospinning jet is not sufficiently dry upon impact. In others, the liquid may penetrate the wet electrospinning jet and forms a film instead of fiber mesh [Kawahara et al 2018]. Kawahara et al (2018) showed that when using wet electrospinning for silk fibroin, a concentration below 5% and above 12.5% resulted in the formation of film on 70% isopropyl alcohol aqueous solution. Other setup consideration may include the selection and placement of counter-electrodes. Having a non-conductive reservoir containment may reduce the incidence of fibers depositing on the reservoir edges. A counter electrode may be submerged in the liquid to attract the electrospinning jet [Kawahara et al 2018].
As coagulation bath
The use of a coagulation bath in electrospinning is very useful for collecting fibers from less volatile solution. Ionic liquid are generally non-flammable which makes electrospinning safer but it is also non-volatile which complicates fiber collection on solid substrate. Miyauchi et al (2010) used a coagulation bath comprising of ethanol and water to collect core-shell fiber of multi-walled carbon nanotube (MWNT) core and cellulose shell. Both materials were dissolved in 1-methyl-3-methylimidazolium acetate ([EMIM][Ac]) ionic liquid for electrospinning. When the electrospinning jet comes into contact with the coagulation bath, the ethanol and water was able to quickly remove the ionic liquid from the fibers. Fibers with diameters ranged from several hundred nanometers to micrometers were collected in the coagulation bath.
It is generally assumed that electrospun fibers collected on a coagulation bath will have a larger diameter compared to the same processing parameters but collected on a dry surface. This is due to solidification of the electrospinning jet upon impact on the non-solvent liquid. However, there are instances where the electrospun fibers collected in the liquid bath showed a smaller diameter compared to a dry surface collector. Prasad et al (2020) in the collection of electrospun poly(vinylidene fluoride) (PVDF) fibers showed that depending on the solvent combination used in the solution, the fibers collected on a water bath may or may not exhibit a larger diameter compared to those collected on grounded aluminum foil. N,N-dimethylformamide (DMF)/acetone was used to prepare the PVDF solution for electrospinning. When the ratio of DMF is half or greater, electrospun PVDF fibers collected on water have a smaller diameter than those collected on grounded aluminum foil. It is presently unclear why this is so although it may be due to the molecular arrangement of the polymer. Electrospun PVDF fibers (DMF:Acetone ratio 7:3) deposited on water were found to have greater β-phase crystals compared to those deposited on aluminium foil. Another possible explanation may be partial dissolution of PVDF deposited on the water as DMF has a higher boiling point than water. With greater DMF concentration, there may be more residual DMF on the surface of the fibers and this may encourage dissolution of PVDF from the fiber surface leading to a smaller fiber diameter.
Using a coagulation bath as a collector introduces another method of modifying the electrospinning process to influence pore formation in the resultant fibers. Phase separation is one of the most common processes that drives the formation of pores in electrospun fibers and three common forms are thermally induced phase separation (TIPS), vapor-induced phase separation (VIPS), and non-solvent induced phase separation (NIPS). Utilising water bath as a non-solvent induced phase separation (NIPS) and it is important to select a solvent that is non-polar. A solvent that is miscible with water will allow near instantaneous entry of water into the jet and precipitation of the polymer. Using polystyrene (PS) and N,N-dimethyl formamide (DMF) as the solvent, the resultant electrospun PS fibers showed no surface pores [Zhu et al 2020]. However, when chlorobenzene (CB) was added into the PS in DMF solution with a CB/DMF ratio of 1:3, porous fiber was produced. This is due to CB being immiscible with water and this encourages phase separation to occur within the fiber. As the electrospinning jet enters the water bath, the water being a non-solvent initiate phase separation with polymer-rich and polymer-poor regions. Nucleation and growth resulted in the formation of numerous, interconnected pores. When the water bath was heated to 40°C, the pore size started to decrease. This is probably due to reduced time available for nucleation.
Coating on electrospun fibers
An advantage of using a non-solvent fluid collector is that an additive can be added into it for direct coating of the deposited fibers. Zheng et al (2014) prepared a wet aqueous suspension of magnesium hydroxide nanoparticles as collector. Cellulose fibers were formed by electrospinning an ionic liquid solvent. Once the fibers coagulate in the aqueous suspension, they were coated with magnesium hydroxide nanoparticles. Im et al (2012) coated fibers with precursor materials by electrospinning directly into a coagulant suspension to create carbon/TiO2 nanotubes. In their setup, poly(vinyl alcohol) (PVA) aqueous solution was directly electrospun into a coagulation bath containing titanium (IV) tetraisopropoxide (TTIP) solution. PVA fibers were coated with TTIP and the resultant composite was heat-treated to form carbon/TiO2 nanotubes.
To fabricate 3D scaffold through sinking of fibers
A non-solvent fluid can be used to collect block electrospun scaffold if the surface tension of the fluid is sufficiently low such that the fibers sink below its surface upon impact. Kostakova et al (2014) showed that with electrospinning of polycaprolactone (PCL) solution, an ethanol concentration of more than 65% is necessary for the fibers to sink below its surface.
Organization of fibers
On a solid substrate, electrospinning typically produces a flat membrane. Attempt to remodel it is very often difficult as the membrane is adhered to the collector's surface and the interconnecting fibers form a stable structure. On a moving fluid such as water with high surface tension, the fibers are lightly adhered to the surface and are transported away before the fibers form a stable structure. Depending on the flow of the water, various structures such as yarn [Teo et al 2007], 3-dimensional scaffold [Teo et al 2008] and single layer fiber membrane has been constructed.
Fibers supported by the fluid present a dynamic platform that allows further reorganization of the fibers. Ma et al (2024) submerged an inverted T shape rod with a magnetic base in a 63% ethanol bath that was placed on a magnetic stirrer. With electrospinning depositing polycaprolactone (PCL) fibers on the surface of the 63% ethanol bath, the rotating rod was able to wind the fibers around the circumference of the rod. To wind the fibers along the length of the vertical rod, the liquid level was progressively raised in the collector tub by transferring liquid from another tank.
To collect stand-alone electrospun membrane
Where collection of stand-alone electrospun membrane is required, deposition of the fibers onto a non-solvent bath enables the collection of the membrane without damaging its surface. On a solid substrate, the fibers may adhere to the collector's surface and the membrane surface will inevitably be damaged during the peeling process. To construct 3-dimensional scaffold, Orr et al (2015) deposits electrospun fibers onto a waterbath before stacking them together. Certain polymers are known to be sticky and peeling the electrospun membrane after depositing them on a solid substrate will certainly damage the membrane. This is where depositing the fibers on a non-solvent bath is recommended. For this reason, Cikova et al (2018) used water for collection of electrospun ethylene vinyl acetate/poly(lactic acid) blends (EVA/PLA). EVA has a tendency to stick on aluminium surface thus the membrane will be destroyed during the removal process. A water bath collector is able to capture pure electrospun EVA fibers. However, the resultant fiber diameter is a few microns thick. By blending PLA into EVA, the fiber diameter may be reduced to about 500 nm. Interestingly, only when EVA ratio was increased to 60:40(EVA:PLA) was EVA detected in the outer fibers.despite PLA being more hydrophobic.
In another use for non-solvent collection of electrospun fibers, Eom et al (2017) were able to construct a well insert with electrospun membrane coated at the top. While a conventional solid collector would require an additional step of peeling the deposited electrospun membrane from the collector surface, Eom et al (2017) used 0.5 M potassium chloride (KCl) solution as the grounded collector by filling the well with it. Since the electrolyte is conductive and electrically grounded, the electrospinning jet preferentially deposits fibers over the surface of the solution. After sufficient thickness was accumulated, the solution can be drained to leave behind the electrospun membrane on the well.
Surface Functionalization
As a non-solvent collector, the fluid selected for collecting the electrospun fibers may be tailored to initiate surface reaction on the fiber. Sodium hydroxide is commonly used for surface hydrolysis of some polymers especially esters and amides bonds. Therefore, aqueous sodium hydroxide may be used to collect fibers where surface chemical treatment is required [Salehi and Bastami 2016]. Ethanol may be added to the solution to reduce the surface tension of the liquid bath so that the deposited fibers would sink below the surface.
Application requirement
For some application, the electrospun structure may be required to be maintained in a wet state. To prevent possible desiccation of cells loaded in a hydrogrel mixture and applied to the electrospun membrane, Xu et al (2013) electrospun the polycaprolactone (PCL)/Pluronic F-127 solution onto a water-bath. Further, since PCL is hydrophobic, a wet membrane may allow better integration with the hydrogel seeping into the pores between the wet fibers. Yang et al (2009) takes advantage of this concept to electrospin PCL/collagen onto cell culture media where cell seeding can be carried out immediately followed by electrospinning another layer of PCL/collagen fibers over the seeded cells. This process is repeated until sufficiently thick layers of fibers with cells were constructed.
Electrospinning and electrospraying of cells simultaneously has the advantage of even distribution of cells throughout the scaffold. However, an important aspect is the percentage of viable cells after the electrospinning and seeding process. Polymers used in the construction of tissue supporting scaffolds mostly dissolve in organic solvents to form the solution for electrospinning and these solvents are often toxic to cells. Schuttler et al (2020) showed that with a liquid collector, cell viability increases significantly compared to a conventional solid substrate collector. When a cell culture media was used in the liquid collector, the culture media has a protective effect both from dilution of any residual solvents, it also possibly prevents the cells from drying out. There is a jump in cell viability from 34% to more than 70% when a solid aluminum collector was switched to a DMEM liquid collector.
Published date: 29 December 2015
Last updated: 17 December 2024
▼ Reference
-
Cikova E, Kulicek J, Janigova I, Omastova M. Electrospinning of Ethylene Vinyl Acetate/Poly(Lactic Acid) Blends on a Water Surface. Materials 2018; 11: 1737
Open Access
-
Eom S, Park S M, Han S J, Kim J W, Kim D S. One-step fabrication of a tunable nanofibrous well insert via electrolyte-assisted electrospinning. RSC Adv. 2017; 7: 38300.
Open Access
-
Im J H, Yang S J, Yun C H, Park C R. Simple fabrication of carbon/TiO2 composite nanotubes showing dual functions with adsorption and photocatalytic decomposition of Rhodamine B. Nanotechnology 2012; 23: 035604.
-
Kawahara Y, Okamura S, Yoshioka T. Structure of regenerated nonwoven silk fibroin nanofiber fabric produced by wet electrospinning. Nippon Silk Gakkaishi 2018; 26: 47.
Open Access
-
Kostakova E, Seps M, Pokorny P, Lukas D. Study of polycaprolactone wet electrospinning process. eXPRESS Polymer Letters 2014; 8: 554.
Open Access
-
Liu J, Liu Q, Ma S, Liang J, Ma X, Fong H. Continuous bundles of aligned electrospun polyacrylonitrile copolymer nanofibers prepared via the flowing water bath and their morphological, structural, and componential variations during the opposite-directional diffusion process. Polymer 2013; 54: 4987.
-
Ma F, Huang X, Wang Y. Fabrication of a Triple-Layer Bionic Vascular Scaffold via Hybrid Electrospinning. Journal of Functional Biomaterials. 2024; 15(6):140.
https://www.mdpi.com/2079-4983/15/6/140 Open Access
-
Miyauchi M, Miao J, Simmons T J, Lee J W, Doherty T V, Dordick J S, Linhardt R J. Conductive cable fibers with insulating surface prepared by coaxial electrospinning of multi-walled nanotubes and cellulose. Biomacromolecules 2010; 11: 2440.
-
Orr S B, Chainani A, Hippensteel K J, Kishan A, Gilchrist C, Garrigues N W, Ruch D S, Guilak F, Little D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015; 24: 117.
-
Prasad G, Liang J W, Zhao W, Yao Y B, Tao T, Liang B, Lu S G. Enhancement of solvent uptake in porous PVDF nanofibers derived by a water-mediated electrospinning technique. Journal of Materiomics 2020 Article in press.
Open Access
-
Salehi M, Bastami F. Characterization of Wet-electrospun Poly (ε-caprolactone)/Poly (L-lactic) Acid with Calcium Phosphates Coated with Chitosan for Bone Engineering. Regeneration, Reconstruction and Restoration 2016; 1: 69.
Open Access
Schuttler K F, Bauhofer M W, Ketter V, Giese K, Eschbach D A, Yenigun M, Fuchs-Winkelmann S, Paletta J R J. Direct incorporation of mesenchymal stem cells into a Nanofiber scaffold - in vitro and in vivo analysis. Sci Rep 2020; 10: 9557.
Open Access
-
Teo W E, Liao S, Chan C K, Ramakrishna S. Remodeling of Three-dimensional Hierarchically Organized Nanofibrous Assemblies. Current Nanoscience 2008; 4: 361-369.
-
Teo W E, Gopal R, Ramaseshan R, Fujihara K, Ramakrishna S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer 2007; 48: 3400-3405.
-
Xu T, Binder K W, Albanna M Z, Dice D, Zhao W, Yoo J J, Atala A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013; 5: 015001.
-
Yang X, Shah J D and Wang H (2009) Nanofiber Enabled Layer-by-Layer Approach Toward Three-Dimensional Tissue Formation. Tissue Engin. A 15 945-956.
-
Zheng Y, Miao J, Maeda N, Frey D, Linhardt R J, Simmons T J. Uniform nanoparticle coating of cellulose fibers during wet electrospinning. J. Mater. Chem. A 2014; 2: 15029.
-
Zhu L, Zaarour B, Jin X. Direct generation of electrospun interconnected macroporous nanofibers using a water bath as a collector. Mater. Res. Express 2020; 7: 015082
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech