Collagen is one of the main structural protein that forms the extracellular matrix (ECM) in our body. The are commonly found in the form of nanofibers and hence the interest in electrospinning which is able to fabricate nanofibers. While electrospun nanofibers is able to mimic the form of ECM, biological cue also has significant influence on the behavior of cells. To construct an ECM that truly mimics natural ECM both in terms of physical structure and biochemistry, there is great interest in electrospinning collagen nanofibers.
Electrospinning is an easy method to produce nanofibers if certain conditions are met. First is that the polymer can be made into a solution form and secondly, the molecular chain is sufficiently long to form chain entanglement. While there have been some success in electrospinning other solution that does not meet these conditions, those generally requires more stringent optimization of parameters to produce nanofibers. For collagen, electrospinning pure collagen fibers has been a challenge until some fluoro-organic solvents, 1,1,1,3,3, 3-hexafluoro-2-propanol (HFIP) and trifluoro-ethanol (TFE) have been found to be able to dissolve collagen and provide the right viscosity for electrospinning nanofibers. While acetic acid has traditionally been used to solubilize collagen, electrospinning collagen dissolved in acetic acid is generally challenging.
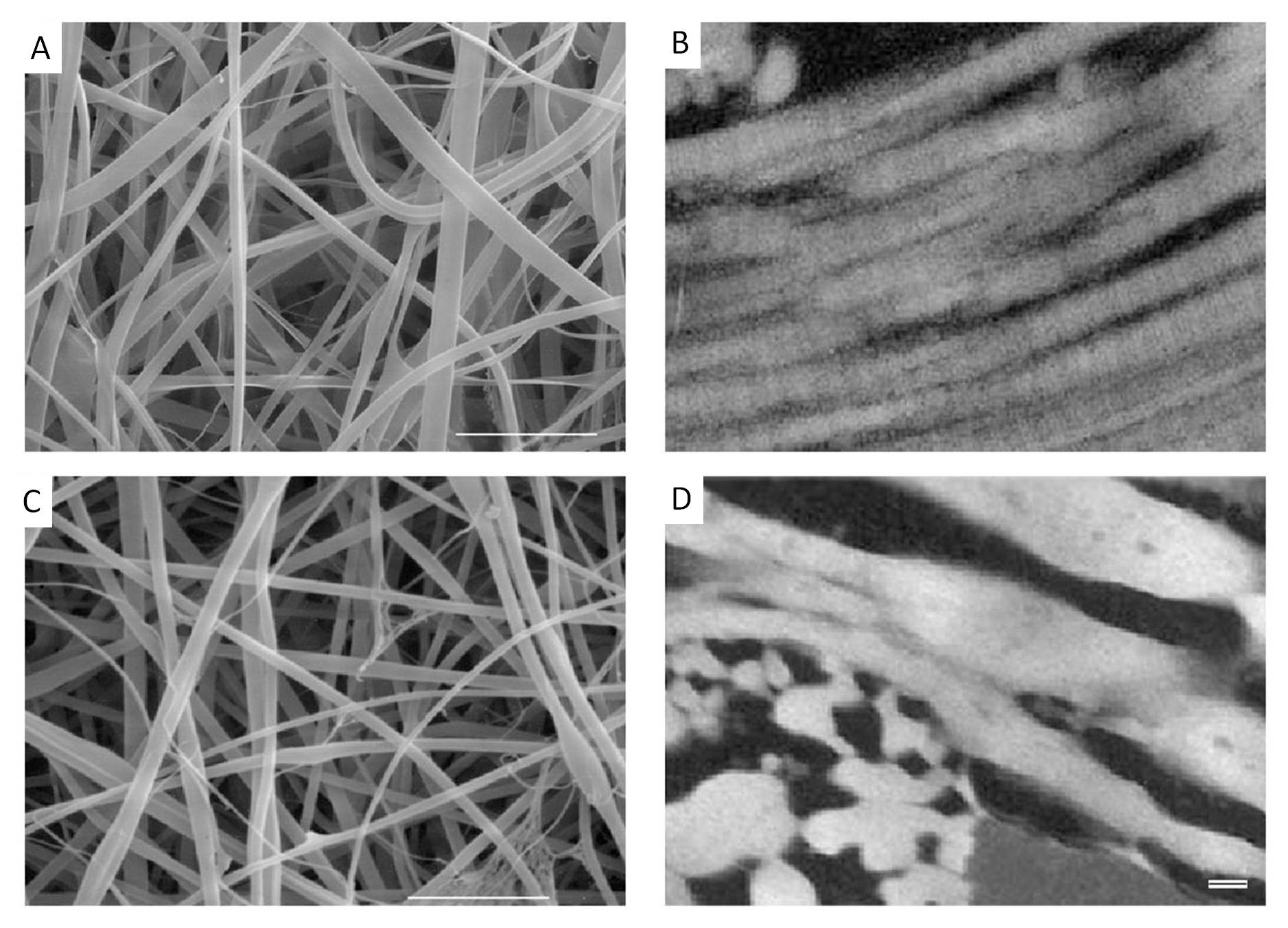
It was in 2002 when Matthews et al (2002) reported the first successful electrospinning of pure Type I and Type II collagen nanofibers using HFIP. They also showed the retention of the distinctive 67 nm banded structure of native collagen in their electrospun nanofibers. Since that publication, other researchers have also reported similar success in electrospinning pure collagen type I nanofibers using the same solvent. Other types of collagen such as type III [Matthews et al 2002] and type IV [Barnes et al 2007] have also been electrospun using the same solvent. While there are several studies on electrospun collagen type I, there had not been any reported observation of the 67 nm banded structure. This has led to some suspicion that at least in most cases, electrospun collagen has lost its native structure. Zeugolis et al (2008) did a detailed study to find out whether electrospun collagen still retains its structure or has it been turned into gelatin. Their result failed to find evidence that the collagen supramolecule remains intact and their conclusion is the collagen has turned to gelatin [Zeugolis et al 2008]. They attribute the cause of collagen denaturing to the flourinated solvents commonly used to dissolve collagen for electrospinning. It is not until 2011 that Jha et al (2011) showed that electrospun collagen type I (from dissolving collagen in HFIP) does exhibit the 67 nm banding pattern. They also compared various cells response to collagen and gelatin electrospun nanofibers and they showed that cells responded more positively on collagen electrospun nanofibers than gelatin [Jha et al 2011].
Given that there is always a concern about the harmful effect of fluorinated solvents on collagen, a more benign alternative solubilizing agent for electrospinning of collagen will be welcomed. Dong et al (2009) reported the successful electrospinning of collagen using a simple binary mixtures of phosphate-buffered saline (PBS) and ethanol to form the collagen solution for electrospinning. To solubilize collagen in room temperature, they found that the 1:1 PBS/ethanol mixture requires the total salt concentration in the PBS buffer to exceed 5 wt.%. FTIR spectra study suggested that the triple helix structure of the collagen was maintained after electrospinning to form fibers. However, more studies and tests on collagen prepared from this solution would be welcomed. Anuradha (2016) used a mixture of glacial acetic acid:dimethylsulfoxide (DMSO) at a ratio of 93:7 to prepare a 10% weight/ volume solution of collagen type I for electrospinning. The resultant electrospun fibers have an average diameter of about 500 nm. Observation of the collagen electrospun fiber under transmission electron micrographs (TEM) reveals triple helical repeats of 67nm D periodicity banding pattern typical of native collagen. This showed that the ultra-structural integrity of collagen is maintained. It is generally difficult to obtain collagen fibers from electrospinning even with the use of fluorinated solvents. While it has been shown that fibers can be produced even with more benign solution, replicating the results is a challenge. This may be due to the quality, source and consistency of the collagen raw material used in forming the solution for electrospinning. To overcome this issue, Wakuda et al (2018) used core-shell electrospinning to facilitate collagen fiber formation by dissolving collagen in acetate buffer solution to form the core and polyvinyl pyrrolidone (PVP) in acetate buffer solution as the shell. Unlike collagen, PVP readily forms fiber through electrospinning. This helps the formation of fibers with collagen at its core. PBS with 20% ethanol was used to wash away the PVP shell to leave behind pure collagen fibers. Using washing buffer of pH 8.4, percentage of triple helical structure maintained calculated from θ 220 value against native collagen hydrogel was found to be 74%. Gelatin showed almost no helical structure while electrospun collagen from solution prepared by HFP was about 30%. In HFP, it is likely that the molecular chain of collagen were denatured by the strong solvent and further fragmented by the shear stress generated during electrospinning. In an acidic solution, collagen was able to maintains its triple helical structure and the difficulty of electrospinning such a collagen solution was overcomed by using a core-shell electrospinning setup. To wash away the PVP shell, a low-concentration ethanol solution was used as this solution is known to stabilises the triple helical structure by removing the water hydrated on the protein. Therefore, the obtained collagen fibers retained higher structure similar to that of native collagen.
While various forms of collagen has been successfully electrospun, it is important to note that as with most naturally derived polymers, the quality and consistency of the polymers may differ. Therefore, whether the collagen is electrospinnable is very dependent on the processing method. To take advantage of the biological cue provided by collagen, many researchers used blending instead to ensure the formation of nanofibers. Fu et al (2014) showed that a blend of electrospun collagen and poly(l-lactic acid-co-ε-caprolactone) (PLCL) scaffolds were able to provide favourable mechanical and biological properties for vascular tissue engineering through their in vivo studies using a nude mice model. Slower degrading polymers may be used in combination with collagen to increase the duration of support provided by the scaffold. Mechanical strength may also be tailored since pure collagen scaffold may not be sufficiently strong. While the addition of collagen into synthetic polymer has been shown to promote cell adhesion and proliferation in many research, too much of it may be suboptimal. Bertram et al (2017) showed that with T17b murine embryonic endothelial progenitor cells (eEPCs), there is an optimal ratio of collagen to polycaprolactone (PCL) to achieve optimum cell confluence and growth factor secretion on the electrospun scaffold over the first 72 hours. In their study, collagen content of 25% and greater showed significantly greater production of VEGF compared to collagen content of just 5% and pure PCL nanofibers. However, at day 3 scaffold containing 75% collagen has lost its ultrastructure. With the loss of structure, the cells form high density clusters instead of spreading over the surface of the scaffold. With 50% collagen, although the ultrastructure was maintained, the cells were stacked on one another without any organization and were not homogeneously distributed on the surface of the scaffold. Therefore, 25% collagen was proposed to be the optimum blend for PCL electrospun fibers scaffold.
In applications such as medical and food packaging, it is an added advantage if non-toxic solvents are used in the manufacturing process. Chen et al (2024) examined the use of pullulan (PUL) as the supporting material in the electrospinning of collagen. PUL is a microbial and linear polysaccharide and it can be dissolved in acetic acid together with collagen (COL). Further, PUL is able to form hydrogen bonding with collagen molecules for more consistent electrospinning. The resultant COL/PUL electrospun fibers were found to retain 36 % of the triple helix fraction of COL while electrospun COL fibers from using 2.2.2-trifluoroethanol as solvent retained 16% of the triple helix.
A disadvantage of pure electrospun collagen is its relatively weak mechanical strength and fast degradation rate. Premature lost of structural integrity of the scaffold may hinder cell growth and recovery [Bertram et al 2017]. Other than adding synthetic polymers with greater mechanical strength, chemical cross-linking using 1-ethyl-3-(3-dimethyl-aminopropyl)-1-carbodiimide hydrochloride/N-hydroxy succinimide or glutaraldehyde vapor, have been shown to improve mechanical strength [Akhshabi et al 2018] and reduce degradation of electrospun collagen scaffold [Zhong et al 2018]. Glycosaminoglycan (GAG) is a naturally occurring polysaccharides commonly found together with collagen as part of the natural extracellular matrix (ECM) of most tissues. The addition of GAG such as chondroitin sulfate (CS) into collagen solution for electrospinning has shown that the resultant nanofiber scaffold exhibit greater mechanical strength [Akhshabi et al 2018] and slower degradation rate [Akhshabi et al 2018; Zhong et al 2005]. Dhand et al (2018) used quaternary ammonium organosilane (QOS) as a cross-linker for electrospun collagen to alter its mechanical properties and to incorporate anti-bacterial properties. QOS was added to collagen type I solution prior to electrospinning. The resultant QOS/collagen fibers were transferred to a sealed desiccator containing ammonium carbonate to create an alkaline condition which faciliated the hydrolysis of -Si(CH3O)3 groups and initiate the polycondensation of silanol groups and cross-linking of -OH groups in collagen nanofibers. The cross-linked QOS/collagen nanofibrous mat showed significant increase in elastic properties with corresponding decrease in mechanical strength and stiffness. The presence of 0.1%QOS in electrospun collagen nanofiber demonstrated effective inhibition of gram-positive bacterial strains including S. aureus/MRSA. This amount of QOS was also shown to be non-cytotoxic to dermal fibroblast and osteoblast although higher amount of QOS (0.5%) was found to be cytotoxic. Therefore 0.1% QOS in collagen electrospun fibers has the potential for use in the development of medical scaffold where infection control is important.
Published date: 19 September 2017
Last updated: 06 August 2024
▼ Reference
-
Akhshabi S, Biazar E, Singh V, Keshel S H, Geetha N. The effect of the carbodiimide cross-linker on the structural and biocompatibility properties of collagen-chondroitin sulfate electrospun mat. International Journal of Nanomedicine 2018; 13: 4406.
Open Access
-
Anuradha E. Development and characterization of biocompatible electrospun nanofibrous matrices for cardiac tissue engineering. Sri Ramachandra University. PhD Thesis 2016.
-
Barnes C P, Sell S A, Boland E D, Simpson D G, Bowlin G L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Advanced Drug Delivery Reviews 2007; 59: 1413.
-
Bertram U, Steiner D, Poppitz B, Dippold D, Kohn K, Beier J P, Detsch R, Boccaccini A R, Schubert D W, Horch R E, Arkudas A.
Vascular Tissue Engineering: Effects of Integrating Collagen into a PCL Based Nanofiber Material. BioMed Research International 2017; 2017: 9616939, 11 pages.
Open Access
-
Chen J, Li J, Li Y, Wu S. Fabrication and characterisation of collagen/pullulan ultra-thin fibers by electrospinning, Food Chemistry: X 2024; 21: 101138.
Open Access
-
Dhand C, Balakrishnan Y, Ong S T, Dwivedi N, Venugopal J R, Sriram Harini S, Leung C M, Wei Low K Z, Loh X J, Beuerman R W, Ramakrishna S, Verma N K, Lakshminarayanan R. Antimicrobial quaternary ammonium organosilane cross-linked nanofibrous collagen scaffolds for tissue engineering. International Journal of Nanomedicine 2018: 13; 4473.
Open Access
-
Dong B, Arnoult O, Smith M E, Wnek G E. Electrospinning of Collagen Nanofiber Scaffolds from Benign Solvents. Macromol. Rapid Commun. 2009; 30: 539.
-
Fu W, Liu Zm Feng B, Hu R, He X, Wang H, Yin M, Huang H, Zhang H, Wang W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomedicine 2014; 9: 2335.
Open Access
-
Jha B S, Ayres C E, Bowman J R, Telemeco T A, Sell S A, Bowlin G L, Simpson D G. Electrospun Collagen: A Tissue Engineering Scaffold with Unique Functional Properties in a Wide Variety of Applications. J. Nanomat. 2011, 348268.
Open Access
-
Matthews J A, Wnek G E, Simpson D G, Bowlin G L.Electrospinning of collagen nanofibers. Biomacromolecules 2002; 3: 232.
-
Matthews J A, Boland E D, Wnek G E, Simpson D G, Bowlin G L. Electrospinning of Collagen Type II: A Feasibility Study. Journal of Bioactive and Compatible Polymers 2003; 18: 125.
-
Wakuda Y, Nishimoto S, Suye S I, Fujita S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Scientific Reports 2018; 8: 6248.
Open Access
-
Zeugolis D I, Khew S T, Yew E S, Ekaputra A K, Tong Y W, Yung L Y, Hutmacher D W, Sheppard C, Raghunath M (2008) Electro-spinning of pure collagen nano-fibres-just an expensive way to make gelatin? Biomaterials, 29 pp. 2293-2305
-
Zhong S, Teo W E, Zhu X, Beuerman R, Ramakrishna S, Yung L Y. Formation of collagen-glycosaminoglycan blended nanofibrous scaffolds and their biological properties. Biomacromolecules. 2005; 6: 2998.
▲ Close list
 ElectrospinTech
ElectrospinTech