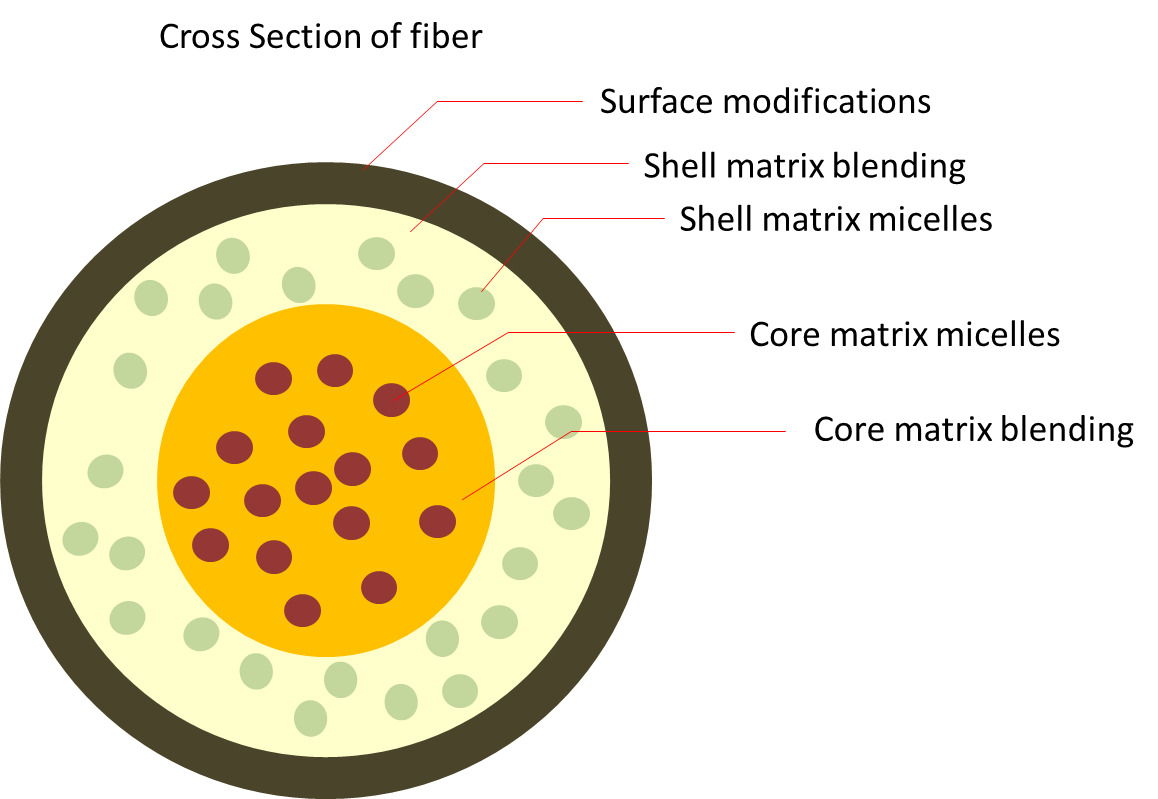
The flexibility of incorporating active substance into electrospun fibers makes it possible to combine drugs for treatment. Combination of drugs or active substances is increasingly being used for treating diseases such as AIDS and other drug resistant infections. This technique reduces the ability of the virus or bacteria to mutate or build up defence against treatment. There are a few ways in which different active substances can be incorporated into electrospun fibers.
Blending multiple active substances into single fiber
Methods to deliver multiple drugs using electrospun fibers have been developed to take advantage different effects of each drug. Yang et al (2014) used a combination of blending micelle encapsulated circumin and hydrophilic doxorubicin hydrochloride (Dox) into polyvinyl alcohol (PVA) solution for electrospinning. The resultant fibers showed inhibitory effects on HeLa cells (Cervical cancer cells) which otherwise proliferate well on pure PVA fibers. Depending on the drugs, combining drugs in the same fiber polymer matrix may or may not affect its individual release rate. Song et al (2012) showed that with fluorescein (FLU) and rhodamine B (RHB) mixed into electrospun poly(lactic-co-glycolic acid) (PLGA) fibers, their release rate were independent of one another.
Different drugs combination may also influence the release rate of the drugs. Blakney et al (2014) found that when tenofovir (TFV) and levonorgestrel (LNG) were blended together and electrospun in polyvinyl alcohol (PVA) solution, the release rate of TFV was slower than when TFV alone was loaded into the PVA fiber matrix. This has been attributed to the more hydrophobic nature of LNG in the matrix which reduces the release of TFV.
In some cases, drugs with different release rates may be incorporated into the electrospun fiber matrix. Wei et al (2021) used poly(N-isopropyl acrylamide- N-Methylol acrylamide-acrylic acid) (PNIPAm-NMA-Ac) as the material for drug loading and electrospinning into fibrous membranes. Two antimicrobial drugs, gatifloxacin hydrochloride (GH) and silver nanoparticles (Ag) were incorporated in the electrospun fibrous membrane. GH is by blending and Ag is by post-spinning reduction of Ag salt which was loaded into the solution prior to electrospinning. With this combination and immersed in PBS solution at temperature of 37 °C, GH had a burst release profile with 72% released in the first 40 min while Ag showed a burst release of 32% in the first 20 min and a more sustained release to 64% over 3 h. As PNIPAm-NMA-Ac is a thermal sensitive material, lower temperature of 20 °C results in reduced drug release. Ag at the lower temperature showed only one stage release profile with a maximum of only 26% released in 50 min. For GH, a maximum amount of 38% was released in 40 min.
Where multiple drugs are used together, a blended mixture of polymers may also be used to influence the release rate of each drug. Thao et al (2021) loaded dexamethasone (Dex) and silver sulfadiazine (AgS) into electrospun small intestinal submucosa (SIS)/poly(ε-caprolactone-ran-l-lactide) (PCLA) (5:1) membrane. In an in vivo drug release test, Dex, which is more water soluble than AgS, saw a rapid release on the first 3 days (40%) followed by linear release of the remaining amount over 14 days. For AgS, 37% was released on the first 3 days with little release over 21 days. The more water soluble Dex may have greater affinity towards SIS in the polymer mixture which has a higher degradation rate. Since AgS is poorly water soluble, more of it may get trapped in PCLA and the slow degradation of PCLA would have slowed its release after the initial burst release. Degradation test using collagenase showed the SIS sheet completely degraded after 1 day, electrospun SIS/PCLA (5:1) membrane showed 50% degradation in a day but remained the same for 3 days and electrospun PCLA membrane showed no degradation. For a membrane made of SIS and PCLA, the initial fast degradation is likely due to the degradation of SIS and the degradation rate slows down significantly due to slow degradation of the remaining PCLA.
Incorporating fibers with different active substances
Since electrospun fibers are very fine and can be uniformly dispersed to form a membrane, active substances can be loaded into separate solution and electrospun to form a membrane that contains individual fibers with different active substances. Sudhakaran et al (2015) used this technique to create a membrane containing polycaprolactone (PCL)/TiO2 fibers and PCL/gentamicin fibers. The fibrous membrane containing the mixture was tested against methicillin-resistant Staphylococcus aureus (MRSA). Their results showed that membrane containing both TiO2 and gentamicin has the largest zone of inhibition. Electrospun fibers each containing different drugs may also be electrospun separately to form layers of fibers with different drugs. However, the release rate of the drugs is more dependent on the interaction between the drug and the polymer matrix than the method of combining drug loaded fibers [Blakney et al 2014].
Li et al (2018) created a multi-functional wound dressing by creating a double layer electrospun membrane. The inner layer that comes into contact with skin was made of electrospun chitosan for its biocompatibility and and intrinsic antibacterial nature. Electrospun polycaprolactone (PCL) fibers were used as the outer layer to instill mechanical strength to the membrane. Anti-bacterial compounds, lidocaine hydrochloride (LID) and mupirocin was added to chitosan and PCL fibers respectively. The release profiles of LID and mupirocin were very different. 66% of LID was released in the first hours followed by gradual release to 85% in the following 6 hrs. Mupirocin showed an initial release of 57% in the first 6 hrs followed by sustained release of another 30% over the next 5 days. Therefore it can be seen that LID was released very quickly during the initial phase while mupirocin was able to maintain a gradual release for the remaining half of its load after the first 6 hrs. Slower release of mupirocin was attributed to stronger chemical linkage with PCL molecules. Such drug release profile is clinically relevant to achieve therapeutic concentration of the drug in minimal time.
Core-shell fibers
Co-axial electrospinning is a good method to separate carriers for different drugs on a single fiber. Since each solution feed enters the electrospinning spinneret from independent solution reservoir, the composition can be tailored to each drugs' requirement. A core-shell fiber will be formed upon collection. Zhao et al (2019) investigated the delivery of dexamethasone (DEX) and bone morphogenic proteins 2 (BMP2) in electrospun core-shell fibers and their influence on MSC differentiation. The shell material is made of Zein with DEX and the core material is made of poly-L-lactic acid (PLLA) and BMP2. In vitro study of the drug release rate showed that the release of the drugs was slower when both drugs are present compared to single drug in respective core or shell of the core-shell fiber. DEX in Zein as the shell showed a burst release profile as Zein dissolves in the PBS and releases the drug quickly. In contrast, BMP in PLLA at the core showed a more gradual release characteristic as the Zein shell shield the inner core from direct contact with PBS. When both drugs are present in the core-shell fiber, the reduced release rate of each drug may be due to interaction between the two at the interfaces which slows down their release rate. Chen et al (2015) blended DOX loaded core-shell structured nanoparticles and indomethacin(MC) into a solution of poly(ε-caprolactone) and gelatin before electrospinning to form drug loaded fibrous mat. This mat is implanted on the tumor of rat tumour model. The study showed significant reduction in the tumor size with the multi-drug loaded nanofibrous mat after 18 days but the tumor size increases for rat that is injected with pure DOX and DOX loaded core-shell structured nanoparticles without the nanofiber carrier mat. This shows that the electrospun mat is very effective in localized and targeted delivery of the anti-cancer drugs.
Published date: 31 May 2016
Last updated: 22 January 2022
▼ Reference
-
Blakney A K, Krogstad E A, Jiang Y H, Woodrow K A. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. International Journal of Nanomedicine 2014; 9: 2967.
Open Access
-
Chen Y, Liu S, Hou Z, Ma P, Yang D, Li C. Multifunctional Electrospinning Composite Fibers for Orthotopic Cancer Treatment in Vivo. Nano Res. 2015; 8: 1917.
-
Li X, Wang C, Yang S, Liu P, Zhang B. Electrospun PCL/mupirocin and chitosan/lidocaine hydrochloride multifunctional double layer nanofibrous scaffolds for wound dressing applications. International Journal of Nanomedicine 2018; 2018: 5287.
Open Access
-
Sudhakaran M, Ghouse S S, Nandagopal S, Jagadeeshan S. Fabrication and characterization of electrospun poly(ε-caprolactone) / TiO2 nanocomposite membranes with synergistic antibacterial property with gentamicin against MRSA. International Journal of Scientific & Engineering Research 2015; 6: 1638.
Open Access
-
Thao NTT, Lee S, Shin GR, Kang Y, Choi S, Kim MS. Preparation of Electrospun Small Intestinal Submucosa/Poly(caprolactone-co-Lactide-co-glycolide) Nanofiber Sheet as a Potential Drug Carrier. Pharmaceutics. 2021;13(2):253.
Open Access
-
Wei Z, Yang J, Long S, Zhang G, Wang X. Smart and in-situ formation electrospun fibrous membrane for the control of antimicrobial efficacy. Smart Materials in Medicine 2021; 2: 87.
Open Access
-
Yang G, Wang J, Li L, Ding S, Zhou S. Electrospun Micelles/Drug-Loaded Nanofibers for Time-Programmed Multi-Agent Release. Macromol. Biosci. 2014; 14: 965.
-
Zhao H, Ma Y, Sun D, Ma W, Yao J, Zhang M. Preparation and Characterization of Coaxial Electrospinning rhBMP2-Loaded Nanofiber Membranes. Journal of Nanomaterials 2019; 8106985.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech