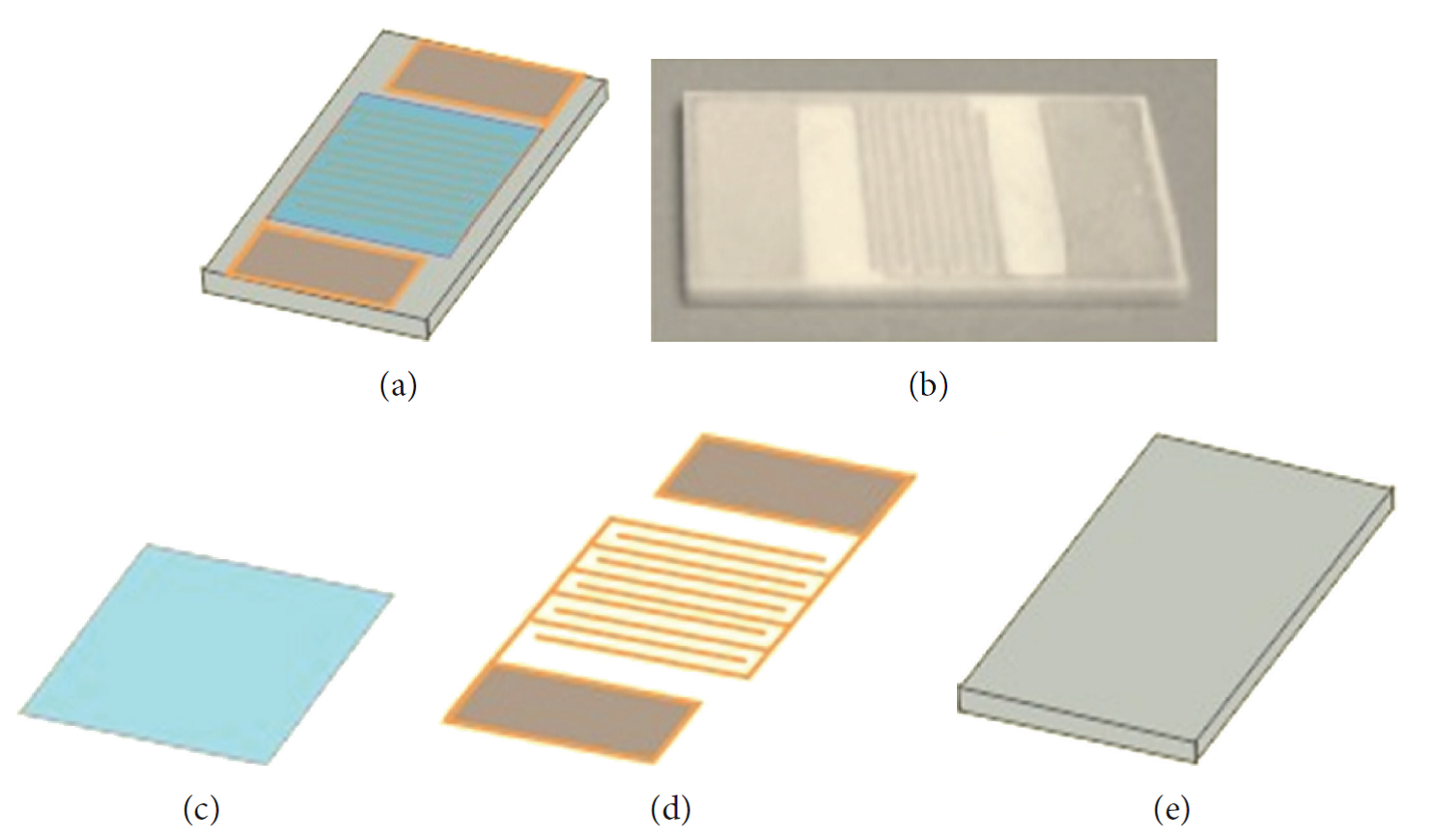
Gas detectors are widely used in industrial and environmental air monitoring. There are many investigations for the use of electrospun fibers as gas sensors [Ding et al 2009]. The ease of fabricating composite fibers and inorganic nanofibers and the variety of materials which it may be constructed from also makes this fiber spinning method popular for developing sensors. High surface area of electrospun nanofibers makes it highly attractive for use as gas sensor as a result of its greater sensitivity. Therefore, electrospun material has been developed for sensing various gas and volatile organic compounds (VOCs). Resistivity changes in the electrospun material is the most commonly used form of signal output. Changes in the materials resistivity arises from adsorption of the gas molecules and this can be easily measured using various electrometers. Ease of fiber deposition on electrodes connected to the electrometer also makes this method of detection attractive.
Physical Characteristics
As resistivity sensor, high surface area alone is not sufficient for its good performance. Fiber continuity is also important for transmission of electrons for faster response and recovery. Chen et al (2013) compared the performance of electrospun Co-doped SnO2 nanofibers and nanosphere for detection of methane. Although nanosphere has a larger surface area, nanofiber based sensor showed better gas response, higher saturated detection concentration, and quicker response-recovery time. In the use of tungsten oxide (WO3) as acetone sensors for the non-invasive diagnosis of diabetes, Ghosh et al (2023) also found better performance of electrospun derived WO3 nanofibers compared to nanoparticles form. WO3 fiber sensor (WFS) has a higher sensitivity of 90% at a lower operating temperature of 150 °C compared to particle-based sensor (WPS) with 84% sensitivity at 200 °C. The response and recovery time for WFS was faster at 10 s and 40 s respectively compared to 18 s and 90 s for WPS. Better performance of WFS has been attributed to higher porosity, charge confinement and electron transfer of a one-dimensional (1D) structure and exposed highly reactive (002) plane with better crystallinity. In the production of WFS, polyvinylpolypyrrolidone (PVP) was added to ammonium metatungstate hydrate solution for electrospinning into fibers. During the sintering process, PVP interacts with the ammonium tungstate surface causing the stabilization of the (002) facet which leads to better crystallinity and exposure of the (002) plane. This reactive plane facilitates more chemisorbed oxygen molecules at a lower temperature which improves the sensitivity of WFS.
Increasing the surface area of the nanofibers will increase the sensitivity of the sensor and this may be brought about by introducing micropores and mesopores on the fiber. Kang et al (2011) used H3PO4 solutions as chemical activation agents to form micropores and mesopores on carbonized electrospun polyacrylonitrile (PAN) fibers. The treated nanofibers sensor showed a six-fold increase in sensitivity to NO gas and response time reduction by 83%. However, it must be noted that while increasing the pores on the fiber may improve sensitivity, it may reduce the recovery rate of the sensor.
Presence of surface defects on the nanofibers have also been shown to be detrimental to its recovery time as it leads to slower desorption of trapped volatile compound molecules. In a study by Wu et al (2009) using ZnO nanowires, the recovery of the sensor was found to be affected by the presence of surface cracks. Calcination at higher temperature reduces the fiber diameter but increases the presence of surface crack on electrospun ZnO. Increased surface area due to the presence of crack increases the sensitivity to 89% for 10 ppm at higher calcination duration of 7h compared to only 32% at shorter calcination duration (5h). However, the recovery duration was much longer for fibers that undergo calcination at the higher temperature.
Material Selection
In general, any materials that showed a change in resistivity when exposed to the VOCs can be used as a resistivity sensor and its sensitivity improved by having it in the form of nanofibers. Wu et al (2009) demonstrated the feasibility of ZnO nanowires for ethanol detection at 220 °C A response time of 19s were recorded for the sensor with 89% sensitivity for ethanol concentration of 10 ppm and up to 94% sensitivity for concentration of 600 ppm. Instead of ZnO, Feng et al (2012) used In2O3 and In2-x Nix O3 for ethanol sensing. At optimum sensing temperature of 180 °C, the response and recovery rate was <3 s and <2 s respectively which is much faster than ZnO.
Doping
Electrospun fibers may be doped with reactive compounds to facilitate adsorption of specific gas. The use of a dopant depends on the characteristic of the gas and the sensor. Zhang et al (2014) used polyaniline (PANi) doped with (+)-camphor-10-sulfonic acid (HCSA) for ammonia detection while undoped PANi was used for nitrogen dioxide (NO2 sensing. HCSA doped PANi fibers exhibits p-type semiconductor characteristic which is suited for detecting electron-donating species such as NH3. NO2 is electron-withdrawing instead of electron-donating thus itself is able to act as a dopant to increase charge carrier concentration of p-type PANi. The fiber sensor exhibited resistance up to 60 fold for ammonia and five orders of magnitude for NO2.
Anwane and Kondawar (2018) used an electrospun poly(methylmethacrylate) (PMMA) fiber base for coating of polyaniline (PANi) over its surface to detect HCl and NH3 gases. In the presence of HCl, protonation of PANi where the HCl molecule donates H+ ions to increase the number of positively charged carriers on PANi, reduces the sensor's electrical resistivity. The reverse happens when the sensor is exposed to NH3 gases. In this case, the NH3 molecule takes up a H+ ion from PANi to form NH4+ which increases the sensor's electrical resistivity. The sensor sensitivity was found to increase with increasing concentration of HCl as well as NH3 gas. The response and recovery time for HCl and NH3 were found to be different. For HCl the response and recovery time for the membrane was found to be almost the same indicating similar adsorption and desorption of HCl molecules. However for NH3, the response time was fast but the recovery time was found to be very slow. This will make the sensor less suitable in application where continuous monitoring of NH3 is required.
Doping may be used to improve the selectivity of the sensor to a mixture of compounds. Liu et al (2011) showed that by doping ZnO nanofibers with Co, the resultant nanofiber was able to distinguish acetone and ethanol/methanol from a complex mixture of vapors by their response especially at the optimum sensing temperature of 360 °C. At this temperature the divergence in the response from acetone and ethanol is the greatest. Doping ZnO with Co further differentiates the response by reducing the optimum operating temperature for ethanol with the optimum operating temperature for acetone unaffected. In sensor applications, doping may be used to improve the performance of inorganic oxides. The additional ion may inhibit the grain growth of the main inorganic compound. Reduced grain size would increase the surface area hence providing more adsorption sites. Having the second ion may also influence the valence band and electron-hole recombination. Fan et al (2021) constructed an acetone sensor using electrospun inorganic nanofibers. They compared the performance of In2O3/NiO composite nanofibers with pure NiO nanofibers. The In2O3/NiO composite nanofibers response to 50 ppm acetone was more than 10 times greater than pure NiO nanofibers. The minimum detection limit of In2O3/NiO composite nanofibers was 10 ppn while pure NiO nanofibers was 100 ppb. The improved performance of In2O3/NiO composite nanofibers over pure NiO nanofibers was attributed to smaller grain size and adjustment of the heterojunction. NiO is a p-type semiconductor while In2O3 is a n-type metal oxide. The difference in the Fermi energy level of NiO and In2O3 encourages the electrons from the conduction band of In2O3 to be transferred to the valence band of NiO. This creates a hole depletion layer on NiO resulting in an increase of the resistance. Reaction of acetone gas on the surface of In2O3 causes more electrons to be transferred from the In2O3 side to the NiO which further widens the hole depletion layer and increasing the resistance. Such changes in the resistance of the composite nanofibers is translated into a higher acetone-sensing response.
Addition and ratio of dopants may also affect the grain size of material that made up the nanofiber. Hu et al (2014) found that nanofibers made of Cd/In molar ratio of 1/1 exhibits larger grain size than ratio of 1/10. They hypothesized that smaller grain size from molar ratio of Cd/In of 1/10 may have contribute to higher response of the nanofiber to formaldehyde.
Electrospun fibers/ Nanoparticles Composite
The additions of nanoparticles to electrospun fibers may potential enhance the performance of the resultant sensor. In this configuration, the nanoparticles are usually the main functional component for detection of the gas while the fibers act as a carrier for holding the nanoparticles and transmission of the electrical signal. Kondawar et al (2014) electrospun polyaniline (PANI)/zinc oxide (ZnO) nanoparticles composite by in-situ polymerization to form a fibrous membrane for sensing ammonia and HCl gas. The response time as given by a change in the membrane resistivity upon exposure to ammonia ranged from 1 to 7 minute while its recovery time was about 14 minute in dry air.
Another way of fabricating fibers/nanoparticles composite is by surface functionalization of the fiber and attachment of the nanoparticles. Zhang et al (2012) first electrospun polyacrylonitrile (PAN) fibers and the resultant membrane was amidoxime functionalized. The amidoxime groups were able to coordinate/chelate with Pd2+ ions which were later reduced to form Pd nanoparticles. Heat treatment was then carried out to carbonize the functionalized PAN fibers. The sensitivity of the functionalized carbon fibers was much more sensitive compared with PAN and carbon fibers.
Functional Layer
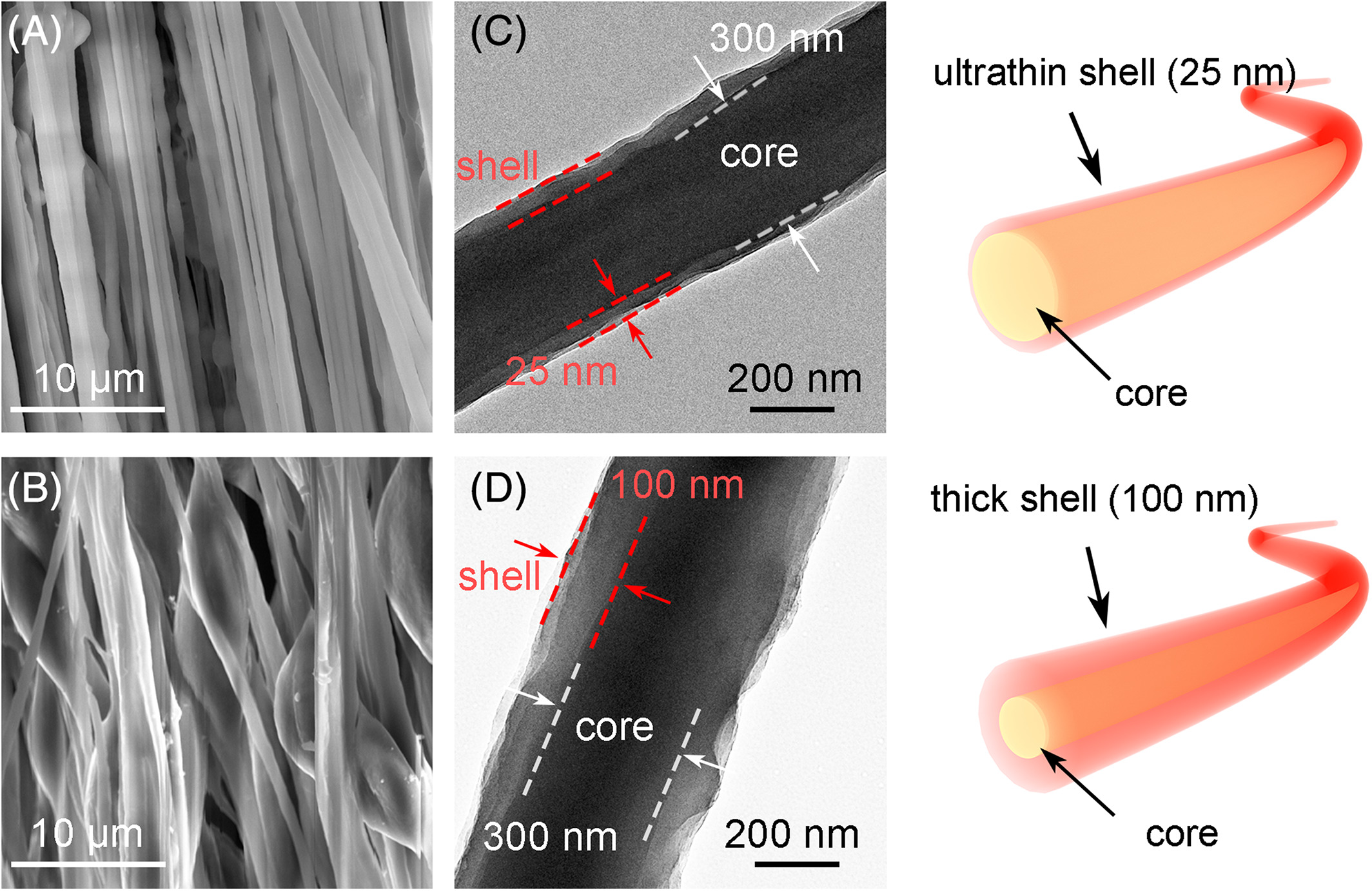
Having the functional molecules on the surface will certainly improve its detection sensitivity. Another consideration is the thickness of the semiconducting functional layer. Having the thickness within the range of Debye length may significantly affect the sensing performance. Liu et al (2019) was able to create a core-shell poly(3,3'"-didodecyl quarter thiophene) (PQT-12) and poly(ethylene oxide) (PEO) fibers with an ultrathin, 25 nm layer of PQT-12 on the surface of the electrospun fibers. The p-type semiconducting PQT-12 was able to show a 13% decrease in current when exposed to only 50 ppb NH3. Using 200 ppb NH3, they found that a PQT-12 concentration of 25% with a thinner (25 nm) layer on the surface of the nanofibers were able to register a significantly better sensing performance than PQT-12 concentration of 66% with thicker 100 nm layer.
Assembly
Portable gas sensors can be constructed with improved sensitivity through the addition of electrospun fibers. This has been demonstrated by Macagnano et al (2014) where they constructed a portable device for sensing asthma based on electrospun fibers through the detection of nitrogen oxide. In their device, the metal electrodes were coated with electrospun titania through sintering of electrospun titania precursor. The titania nanofibers covered electrodes were subsequently dipped into an aqueous dispersion of poly(3,4-ethylenedioxythiophene):- polystyrene sulfonate (PEDOT:PSS) to improve conductivity. The resultant sensor with nanofiber coating has higher sensitivity and faster recovery compared to the original sensor. Ning et al (2021) constructed a H2S sensor by directly coating a ceramic tube with CuO-doped SnO2 precursors using electrospinning and sintering to form CuO-doped SnO2 fibers. By direct coating of the sensor part using electrospinning, it avoided the need for pulverizing the fibers for coating. Comparing the sensors constructed by in situ electrospun fibers on the ceramic tube carrier and coated with pulverized electrospun fibers, the former showed significantly better performance with faster response and recovery. The improved sensing performance has been attributed to the intact fiber network that allows better gas diffusion through the network. In a fractured network, electron transport may be inefficient as there are numerous joints, points of poor contact and dead end paths. With an intact fiber network, the connections to the two electrodes are better as the long fibers are better able to bridge the gap. Therefore, the response and recovery will be much faster.
Published date: 15 March 2016
Last updated: 19 March 2024
▼ Reference
-
Anwane R and Kondawar S. Electrospun Poly(methyl Methacrylate)/Polyaniline Blend Nanofibres with Enhanced Toxic Gas Sensing at Room Temperature. Journal of Physical Science 2018; 29: 101.
Open Access
-
Chen W, Zhou Q, Xu L, Wan F, Peng S, Zeng W. Improved Methane Sensing Properties of Co-Doped SnO2 Electrospun Nanofibers. Journal of Nanomaterials 2013; 2013: 173232. http://www.hindawi.com/journals/jnm/2013/173232/
-
Ding B, Wang M, Yu J, Sun G. Gas Sensors Based on Electrospun Nanofibers. Sensors 2009;9: 1609.
Open Access
-
Feng C, Li W, Li C, Zhu L, Zhang H, Zhang Y, Ruan S, Chen W, Yu L. Highly efficient rapid ethanol sensing based on In2-x Nix O3 nanofibers. Sensors and Actuators B 2012; 166-167: 83.
-
Ghosh P, Manikandan M, Sen S, Devi P S. Some interesting insights into the acetone sensing characteristics of monoclinic WO3. Mater. Adv. 2023; 4: 1146.
Open Access
-
Hu R, Wang J, Chen P, Hao Y, Zhang C, Li X. Preparation of Cd-Loaded In2O3 Hollow Nanofibers by Electrospinning and Improvement of Formaldehyde Sensing Performance. Journal of Nanomaterials 2014; 2014: 431956.
Open Access
-
Kang S C, Im J S, Lee Y S. Improved Sensitivity of an NO Gas Sensor by Chemical Activation of Electrospun Carbon Fibers. Carbon Letters 2011; 12: 21.
Open Access
-
Kondawar S B, Patil P T, Agrawal S P. Chemical vapour sensing properties of electrospun nanofibers of polyaniline/ ZnO nanocomposites. Adv. Mat. Lett. 2014; 5: 389.
Open Access
-
Liu D, Shi Q, Jin S, Shao Y, Huang J. Self-assembled core-shell structured organic nanofibers fabricated by single-nozzle electrospinning for highly sensitive ammonia sensors. Infomat 2019 Article in Press.
Open Access
-
Liu L, Li S, Zhuang J, Wang L, Zhang J, Li H, Liu Z, Han Y, Jiang X, Zhang P. Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sensors and Actuators B 2011; 155: 782.
-
Macagnano A. Bearzotti A, Cesare F, Zampetti E. Sensing Asthma with Portable Devices Equipped with Ultrasensitive Sensors Based on Electrospun Nanomaterials. Electroanalysis 2014; 26: 1419.
-
Ning X, Tang D, Zhang M. Directly electrospinning submillimeter continuous fibers on tubes to fabricate H2S detectors with fast and high response. Nano Materials Science 2021 Article in press
Open Access
-
Wu W Y, Ting J M, Huang P J. Electrospun ZnO Nanowires as Gas Sensors for Ethanol Detection. Nanoscale Res. Lett. 2009; 4: 513.
-
Zhang L, Wang X, Zhao Y, Zhu Z, Fong H. Electrospun carbon nano-felt surface-attached with Pd nanoparticles for hydrogen sensing application. Materials Letters 2012; 68: 133.
-
Zhang Y, Kim J J, Chen D, Tuller H L, Rutledge G C. Electrospun Polyaniline Fibers as Highly Sensitive Room Temperature Chemiresistive Sensors for Ammonia and Nitrogen Dioxide Gases. Adv. Funct. Mater. 2014; 24: 4005.
▲ Close list
 ElectrospinTech
ElectrospinTech