Electrospun scaffold has been widely investigated for use as tissue engineering and regenerative grafts. While electrospun flat mesh is generally good enough for laboratory investigation on cell behavior, an implantable graft generally requires its structure to be closer to the native tissue it is replacing. Dynamic liquid electrospinning has been shown to be very effective in constructing continuous nanofibrous yarn (See link) and three dimensional (3D) scaffold made out of nanofibrous yarn (See link). These structures have been used either as it is or with other structures for various tissue repair.
The 3D nanofibrous yarn scaffold has the characteristics of open, inter-connected yarns which themselves are made out of nanofibers. The inter-mingling yarns create pore sizes that are estimated to be greater than 20 µm by looking at the SEM images. Such large pores allow cells to migration into its depth and the alignment of the nanofibers that form the yarn may potentially guide cell movements along the yarn. In vitro studies have shown that proliferation of the cells on the nanofibrous yarn scaffold is significantly greater than typically flat mesh nanofibrous scaffold. Cell infiltration into the 3D yarn scaffold is also shown to be excellent compared to the flat mesh nanofiber scaffold where cells typically remain close to the surface [Wu et al 2012].
Tendon repair
Native tendons are known to be made out of aligned collagenous nanofibers. Similar structure may also be constructed using the dynamic liquid flow method where the yarn is being drawn onto roller. Xu et al (2013) compared the suitability of random nanofiber mesh, aligned nanofiber mesh and nanofibrous yarn comprising of poly(l-lactide-co-ecaprolactone) [P(LLA-CL)], and natural collagen I for tendon cells. After 14 days of culture, cells were found to infiltrate throughout the nanofibrous yarn scaffold while remains on the surface of the randomly oriented and aligned nanofibrous mesh. This probably influence the number of tendon cells on the various substrates as nanofibrous yarn was found to contain significantly greater numbers than the other nanofibrous substrates. The number of cells on the nanofibrous yarn was also greater than tissue-culture polystyrene plates (TCP) which showed comparable cell quantity to the other nanofibrous substrates. The expressions of type I collagen, type III collagen, decorin, tenascin-C, and biglycan in tendon cells were also found to be significantly greater in nanofibrous yarn compared to the flat nanofibrous mesh after 14 days. In an in vivo study, Xu et al (2014) compared the outcome of implanted nanofibrous yarn scaffolds pre-seeded with tendon-derived stem cells (TDSCs) that were cultured in static condition and under dynamic loading using a rabbit model. Full infiltration of the cells into the scaffold was seen in both static and dynamic culture. Following explantation of the scaffold, both scaffold showed expression of tenogenic differentiation with the dynamic system culture showing greater expression. The mechanical strength of the scaffolds with cultured cells was also better than the control scaffold demonstrating good integration of the secreted collagen with the scaffold.
Peripheral Nerve
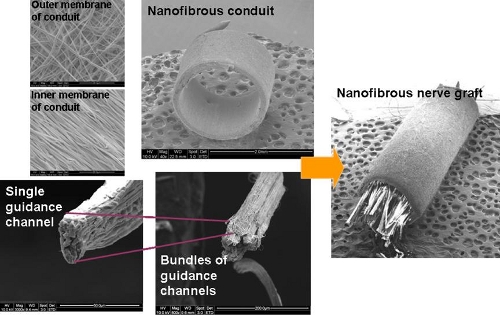
Following a nerve injury, it is essential for the proximal axon to connect with the Bunger bands within 3 to 4 months. For large gaps (greater than 15 to 30 mm), the axon is unable to bridge the gap without any external facilitation. To achieve this, one method is to construct a hybrid structure using nanofibrous yarns as intra-luminal guidance channel within a nanofibrous tube such that axon migration across the gap can be directed. In particular, the nanofibers that form the yarn are aligned longitudinally and the yarn has been demonstrated in other studies to direct cell migration [Wu et al 2012]. An in vivo study based on Wistar rat sciatic nerve demonstrated the potential of the nanofibrous yarn as guidance channel for use in peripheral nerve repair. Comparison was made with empty nanofibrous conduit and autologous graft with several tests demonstrating comparable results between the hybrid nanofibrous structure and autologous graft. Empty nanofibrous conduit generally gives poorer results for most of the tests [Koh 2009].
Adipose tissue carrier
3D nanofibrous yarn scaffold has been demonstrated as a potential carrier for adipose tissues. In a study by Panneerselvan et al (2012), minced adipose tissues are loaded into the 3D nanofibrous yarn scaffold and cultured in vitro to determine the viability of the cells over 4 weeks. At the end of 4 weeks, adipocytes and endothelial cells that were found in the adipose tissues are still surviving in the core of the bioartificial graft (3D nanofibrous yarn scaffold with minced adipose tissue). This contrasts with the intact adipose tissue where most cells at the core are dead. The bioartificial graft was able to remain as a single piece probably due to the minced adipose tissues clinging onto the nanofibrous yarn scaffold through a combination of high surface area of the nanofibers and the adhesion between the fat tissue extracellular matrix (ECM) and the hydrophobic polymer surface. High pore size and porosity of the bioartificial graft probably allows sufficient diffusion of nutrients and oxygen from the surface to its core.
Bone graft
To eliminate the need for open surgery in bone graft applications, injectable bone grafts have been developed. To improve the mechanical strength of injectable collagen type I hydrogel, Liu et al (2014) have incorporated chopped pieces of electrospun nanofibrous yarn. The chopped yarn was made of poly(L-lactide-co-ε-caprolactone) (P(LLA-CL) with length of about 1 mm and mixed with the collagen to give a weight ratio of 1:1. The storage modulus of the collagen hydrogel with chopped nanofibrous yarns was found to be much greater than pure collagen hydrogel. At day 21, hMSC cultured in collagen hydrogel with chopped nanofibrous yarns showed significantly higher ALP activity compared to collagen alone and Helos. Thus, hydrogel with chopped nanofibrous yarns have the potential for use as a injectable system for treatment in osteoporosis or bone fracture.
3D nanofibrous yarn scaffold is generally very soft due to high aspect ratio of nanofibers and low stiffness of polymer. Its inability to maintain structural integrity especially when wet, makes it difficult to use in certain application. This is particularly in bone grafts for bridging gaps where the graft is designed to take on the load. Nevertheless, external mineralization technique such as alternate dipping has been shown to be able to coat the nanofiber yarn with sufficient calcium phosphate particles to give it the adequate stiffness to maintain its structure [Teo et al 2011]. With this, it is possible to use the mineralized nanofibrous yarn scaffold as bone graft.
A preliminary in vivo study using a rabbit ulna segmental defect model has been used to examine the potential use of the mineralized scaffold as bone graft. Using poly-L-lactide/collagen/nHA 3D nanofibrous yarn scaffold, approximately 50% new bone regeneration was seen after 3 months of implantation compared to about 38% for blank control [Ngiam et al 2010]. However, it is important to note that in this model, the bone graft is not supporting the weight of the animal.
Osteochondral Repair
In osteochondral repair, part of the graft lies within the bone below the cartilage region while the other part remains on the surface for cartilage regeneration. To cater for the requirements of both regions, a hybrid graft comprising of two different materials may be used. The bone interface area is typically made out of a harder material while the upper cap is made out of softer material for cartilage regeneration. Liu et al (2014) constructed a nanofiber yarn-collagen type I/hyaluronate hybrid/TCP biphasic scaffold and tested its potential in osteochondral repair using a rabbit model with the defect at the patellar groove of the distal femur. Comparison was made with sponge-cap which is made out of cartilage extracellular matrix components by freeze-drying. Bone marrow derived stem cells which were undifferentiated or differentiated in chondrogenic medium was seeded in both nanofiber yarn cap or sponge cap scaffolds as part of the study groups. Nanofiber yarn cap with differentiated cells seeded showed cell morphology in the regenerated cartilage almost identical to that of native host cartilage. The sponge cap scaffold also showed smooth repaired surface that is well integrated with the host cartilage. However, nanofiber yarn cap showed three-fold greater compressive strength than sponge cap and the presence of GAG in the middle of the defects are low.
Published date: 06 May 2014
Last updated: 02 June 2015
▼ Reference
-
Koh H S. Polymeric Nanofiber Conduits for Peripheral Nerve Regeneration. PhD Thesis 2009, National University of Singaopre.
Open Access
-
Liu S, Wu J, Liu X, Chen DS, Bowlin G L, Cao L, Lu J, Li F, Mo X, Fan C. Osteochondral regeneration using an oriented nanofiber yarn-collagen type I/hyaluronate hybrid/TCP biphasic scaffold. Journal of Biomedical Materials Research Part A 2014 Article in press.
-
Liu W, Zhan J, Su Y, Wu T, Ramakrishna S, Liao S, Mo X. Injectable hydrogel incorporating with nanoyarn for bone regeneration. Journal of Biomaterials Science, Polymer Edition 2014; 25: 168.
-
Ngiam M L M. Differentiation of Bone Marrow Derived Mesenchymal Stem Cells (BM-MSCs) using Engineered Nanofiber Substrates. PhD Thesis, National University of Singapore 2010.
Open Access
-
Panneerselvan A, Nguyen L T H, Su Y, Teo W E, Liao S, Ramakrishna S, Chan C W. Cell viability and angiogenic potential of a bioartificial adipose substitute. Journal of Tissue Engineering and Regenerative Medicine. 2012 (Article in press)
-
Teo W E, Liao S, Chan C, Ramakrishna S. Fabrication and characterization of hierarchically organized nanoparticle-reinforced nanofibrous composite scaffolds. Acta Biomaterialia 2011; 7: 193.
-
Wu J, Liu S, He L, Wang H, He C, Fan C, Mo X. Electrospun nanoyarn scaffold and its application in tissue engineering. Materials Letters 2012; 89: 146.
-
Xu Y, Wu J, Wang H, Li H, Di N, Song L, Li S, Li D, Xiang Y, Liu W, Mo X, Zhou Q. Fabrication of Electrospun Poly(L-Lactide-co-e-Caprolactone)/Collagen Nanoyarn Network as a Novel, Three-Dimensional, Macroporous, Aligned Scaffold for Tendon Tissue Engineering. Tissue Engineering: Part C 2013; 19: 925.
-
Xu Y, Dong S, Zhou Q, Song L, Hou Y, Wu J, Li S, Li T, Li P, Gan Y, Xu J. The effect of mechanical stimulation on the maturation of TDSCs-poly(L-lactide-co-e-caprolactone)/collagen scaffold constructs for tendon tissue engineering. Biomaterials 2014; 35: 2760.
▲ Close list
 ElectrospinTech
ElectrospinTech