Most form of electrospinning requires the polymer to be dissolved in a solvent to form an electrospinnable solution. Unfortunately, most of the solvents used to dissolve polymers for electrospinning are toxic and harmful to the environment. Since the process requires the vaporization of the solvent to form fibers, the released solvent vapors may be hazardous to the operator and the environment unless a non-hazardous solvent is used or the solvent vapors can be safely captured or neutralized prior to release. To reduce environmental impact, it is important to consider the selection of polymers for the targeted application so that less harmful solvents may be used. More efforts may also go into identifying a less harmful solvent and optimizing the electrospinning conditions for spinning fibers.
Certainly, water-based polymers will have the least environmental impact. Unfortunately, most water-based polymers are mechanically weak and degrades quickly in the environment. Despite this limitations, there are applications where water soluble polymers has been used instead of solvent-based polymers. For air filtration, polyvinyl alcohol (PVA) has been electrospun to form filter media membrane [ Kusumaatmaja et al 2016]. Oral rapid drug release carrier may also be made from water-soluble polymer and the high surface area of electrospun nanofibers allows faster release compared to film equivalent [Seif et al 2016]. Water soluble electrospun fibers may also be used as templates for building channels in a matrix or to form tubes since they will be removed [Wu et al 2013]. Lin et al (2014) used green electrospinning to produce nanofibers containing silver nanoparticles (AgNPs) with potential application as antibacterial mat or agent. Poly(vinyl alcohol) (PVA) was selected to be the carrier material as it is water soluble and known to be a good stabilizer of AgNPs which will reduce agglomeration of the nanoparticles. AgNO3 was used as the precursor for AgNPs formation. Both AgNO3 and PVA are water soluble thus a aqueous mixture of the solution can be formulated do electrospinning. Electrospun PVA containing AgNO3 can be heat treated or UV irradiated to convert AgNO3 to AgNPs. The resultant PVA-AgNPs fibrous mat was shown to be effective in inhibiting S. aureus and E. coli. Interestingly, the mat showed almost no cytotoxicity with PVA-AgNPs towards PA317 cell line. However, with PVA-AgNO3, there is a AgNO3 concentration dependent cytotoxicity.
For some water soluble materials, the resultant electrospun membrane was able to maintain its form for longer durations. Stie et al (2022) used a mixture of soy protein isolate (SPI) and polyethylene oxide (PEO) aqueous solution for electrospinning into a nanofibrous membrane. Soy protein isolate is a biodegradable and renewable source, however, electrospinning pure SPI with water to form fibers is still unattainable without the addition of other polymers. By sonication of SPI suspension, they were able to obtain an electrospinnable aqueous solution mixture with 75 wt% SPI to PEO. With this high content of SPI, the resultant electrospun membrane was able to maintain its shape for 24h in water without cross-linking. However, when the SPI content was lower at 60% and below, partial and full disintegration of the membrane was observed in 24h.
Hyaluronan (HA) is a naturally occurring linear polysaccharide that has been used as biomedical scaffolds and skincare products. Due to the difficulty of electrospinning pure (HA), most of its fibers are blended with other polymers thus forming a composite fiber. Komal et al (2024) used a sacrificial carrier polymer, polyethylene oxide (PEO) to facilitate electrospinning of HA into fibers before removing the PEO as both are water soluble. To maintain the integrity of electrospun HA fibers after removal of PEO, the HA/PEO solution pH was adjusted after dissolution in water before electrospinning. In acidic conditions HA dissociation is suppressed and promotes hydrogen bond formation between the HA molecules which makes HA less water soluble. Post electrospinning, the HA/PEO fibers undergo heat treatment of 100 °, quenched to 0 °C using a dry bath and immersed in 0.2 M acetic acid aqueous solution for 10 min so that the HA component becomes insoluble in water due to greater intra- and intermolecular hydrogen bonds. The fibers were rinsed in water to remove PEO to give HA nanofibers.
For materials that are non-water soluble, one way is to use their particulate form to mix into a suspension for electrospinning. This involves dispersing the nano-size particles in a solution containing a water-soluble binding polymer. Gonzalez et al (2021) used a mixture of polyvinyl alcohol (PVA), surfactant and latex copolymer particles for electrospinning. PVA is the template polymer to enable electrospinning and the surfactant is used to disperse the latex copolymer particles. Their results showed that particles of larger diameter resulted in electrospun fibers of larger diameter. For particle sizes 107 nm and 192 nm, they were able to produce smooth fibers but with particle size of 317 nm, fibers with pearl necklace morphology were produced probably due to poor packing. The mono-suspension of these three particles with the template polymer exhibited similar viscosities. When the ratio of the particles to PVA was increased to 71/29 wt.%/wt.%, beaded fibers were obtained across all particle sizes. With the same particles to PVA ratio but with a bimodal particles size mixture consisting of the smallest particles (diameter 107 nm) and largest particles (diameter 317 nm) in equal ratio, the resultant electrospun fibers were much smoother. It was found that with this bimodal particle suspension, the viscosity is significantly higher than their monomodal suspension. This probably facilitated the production of uniform fibers.
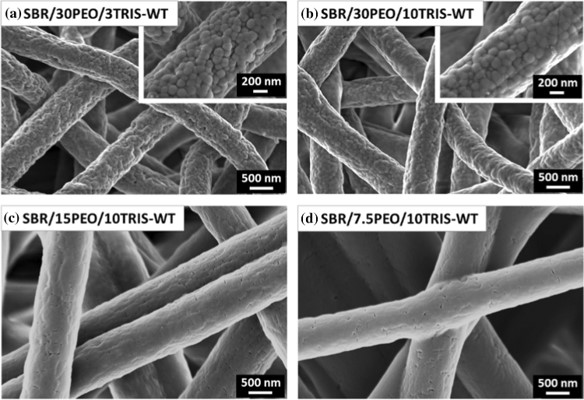
To improve the versatility of electrospun latex particles with PVA as binders, the latex particles may be functionalized prior to blending with PVA and electrospinning. González et al (2023) demonstrated this by using latexes containing CeO2 nanoparticles (NPs), Fe2O3 and quantum dots with PVA as the binder and water as the electrospinning medium. The distribution of the latex particles were uniform along the resultant electrospun fibers. With the NPs bound to the latex particles, there was very little influence by the size and concentration of NPs on the electrospun fiber. While it has been shown that it is possible to use nanoparticles suspension for electrospinning into fibers with the help of electrospinnable polymers, it is advantageous to be able to remove the polymers and retain the fibers. Kianfar et al (2024) electrospun styrene-butadiene rubber (SBR) latex with photo-initiators and polyethylene oxide (PEO) as the electrospinnable binder. Post-electrospinning irradiation at ambient conditions were used to trigger the photo-induced thiol-ene crosslinking reaction. The PEO binder was subsequently washed off with water. Interestingly, despite the SBR latex beads having diameters in the range of 100 to 200 nm, and the electrospun fibers having diameters of 735 to 970 nm, the fibers showed a generally smooth surface both before and after the washing step although in higher PEO concentration SBR latex beads can be seen. With lower PEO concentration, the SBR latex beads were closer and this may encourage greater fusion during cross-linking hence giving a smoother fiber surface.
Cyclodextrin (CD) is an interesting small molecule as it has an internal hydrophobic cavity and external hydrophilic surface. This allows it to form complexes with water insoluble molecules and be soluble in aqueous media. Hence CD is commonly used for drug encapsulation. For electrospinning, CD is often blended into a polymer solution before electrospinning to form fibers. However, under the right concentration and parameters, it is possible to electrospin modified CDs. Topuz et al (2020) demonstrated the ability to electrospin hydroxypropyl-β-CD (HP-β-CD) and HP-γ-CD by dissolving them in water containing quaternary ammonium salt, tetraethylammonium bromide (TEAB), to increase the solution conductivity. Unlike polymers, CDs are oligomers and their solution viscosity does not come from chain entanglements. Electrospinning is possible due to hydrogen bond interactions among CD aggregates at high concentrations. Topuz et al (2020) was able to get beads free fibers at solution concentration of 180 w/v % and diameters less than 400 nm with 1 wt% (with respect to CD) TEAB in the solution. However, such small molecules are sensitive to additives that disrupt the hydrogen bonding which will cause beads formation or breaking up of the spinning jet into spraying instead.
Deep eutectic solvent (DES) is a relatively young class of ionic liquids which has properties that make it safer and greener than conventional volatile organic solvents. It is non-volatile and has the potential to replace organic solvents for dissolution of polymers and extraction of certain ions and molecules. Khatri et al (2020) demonstrated the use of DES based on Quaternary salt (Choline chloride) as a hydrogen bond acceptor (HBA) and (Furfuryl alcohol) as a hydrogen bond donor (HBD) for the dissolution and electrospinning of Zein. By dissolving Zein in Choline chloride (HBA) and Furfuryl alcohol (HBD) with a ratio of 1:2 and a concentration of 45%, Khatri et al (2020) was able to produce beads-free Zein fibers using electrospinning (Zein-DES). The average diameter of the fibers was 350 nm which is smaller than fibers produced by electrospinning of Zein dissolved in ethanol at a concentration of 25% with a diameter of 550 nm (Zein-C). Electrospun Zein-DES was also found to be more hydrophilic than Zein-C. This may be attributed to greater -NH and -OH on the surface of the nanofibers resulting from the protonation by DES. The same -NH and -OH reactive groups on the surface also makes it faster and more efficient at dye removal.
Polymer that is water-soluble will be challenging to use under most environmental condition. To increase the application of water soluble polymers, it is necessary to render it water insoluble. Polymer for electrospinning may be modified such that in situ process or post electrospinning process may be used to transform the fibers into insoluble form. One concept is to incorporate photo-crosslinkable moieties into the base polymer molecule. UV treatment may be carried out during electrospinning or post electrospinning to cross-link the molecular chain thereby making the fibers insoluble. Zhang et al (2017) bonded methacrylic groups on zein and the modified zein remains soluble in ethanol. An electrospinnable solution of the methacrylated-zein (m-zein) was prepared using ethanol aqueous solution concentration of 50 - 80%. During electrospinning, the jet was irradiated by UV light. Post-electrospinning irradiation was carried out for a further 5 minutes to ensure sufficient cross-linking of the fibers. The treated fibers were found to be stable in water and ethanol solution and therefore has the potential to be used in aqueous environment.
As air filters, hydrophilicity of polyvinyl alcohol (PVA) makes it unsuitable for use under a high humidity environment. Liu et al (2022) constructed a superhydrophobic membrane with electrospun polyvinyl alcohol(PVA)/Eugenol (Eo) fibers and electrosprayed ethyl cellulose (EC)/Eugenol (Eo) beads. PVA was dissolved in water heated to 95 °C. Tween-80 emulsifier was added to the solution to form an emulsion with Eo. EC was dissolved in an acetic acid/ethanol for electrospraying. Eo is a hydrophobic aromatic compound which increases the hydrophobicity of electrospun PVA fibers and electrosprayed EC beads. By electrospraying a layer of EC/Eo beads on the base layer of electrospun PVA/Eo fibers, the water contact angle of the PVA membrane increases from 142.8 to 151.1°. The composite membrane at optimum electrospinning and electrospraying duration showed a low filter pressure drop of 168.1 Pa with high filtration efficiencies of 99.74 and 99.77% for PM1.0 and PM2.5 respectively. The respective quality factors were found to be 0.0351 and 0.0358 Pa-1. In a high relative humidity condition of 90%, there is a slight decrease in filtration efficiency from 99.95 at 15% to 99.67%.
There are several methods to cross-link water soluble polymers such that fibers are stable in water. UV irradiation is an effective method which does not require the use of any chemicals. Faria et al (2017) was able to use UV irradiation on electrospun polyvinylpyrrolidone (PVP) to make it insoluble in water. The fibrous membrane will swell in water which may limit its range of applications. Nevertheless, Faria et al (2017) used colloidal electrospinning to produce PVP fiber as template to incorporate thermoresponsive poly-(N-isopropylacrylamide) (PNIPAAm)-based microgels into the composite membrane for potential use as controlled drug release agent.
A freeze thawing process may be used for physical cross linking of certain polymers and it does not require the addition of any potentially harmful cross-linking agents. Nagakawa et al (2020) demonstrated the feasibility of using freeze thawing to improve the stability of electrospun polyvinyl alcohol (PVA) and glycerol (Gly) nanofibers in water. Freeze thawing improves stability of PVA by encouraging the formation of inter- and intramolecular hydrogen bonds and crystallites during the freezing process where ice crystal formation aggregated the PVA chains. Gly addition was also found to improve stability of electrospun PVA chains by forming more hydrogen bonds in the crystal structures. However, only freeze thawed electrospun PVA/Gly nanofibers were found to be stable in water. Although freeze thawing of electrospun pure PVA nanofibers showed approximately the same level of crystallinity improvements as PVA/Gly nanofibers, only PVA/Gly nanofibers were stable in water which showed the importance of hydrogen bonding between PVA and Gly molecules.
The ideal green electrospun fibers would be one where the material is from a natural source and the material can be dissolved in water to form the solution for electrospinning. Cecone et al (2022) demonstrated the feasibility of electrospinning maltodextrin-based microfibers. Maltodextrins are water-soluble, high molecular weight polysaccharide molecules derived from starch hydrolysis. Using maltodextrin of molecular weight ranging from 20 kDa to 272 kDa, fibers can be produced from electrospinning at concentrations of 50 wt% to 62.5 wt% with higher concentrations needed for lower molecular weight maltodextrins. Since the resultant microfibers are water soluble, citric acid was added as a cross-linking agent. Citric acid was added to the maltodextrin solution for electrospinning. The carboxylic groups on citric acid molecules were able to bind with the hydroxyl groups on maltodextrins through condensation reaction resulting in ester formation. This cross-linking process is initiated by heat treatment of the electrospun fibers at 180 °C. It was found that higher molecular weight maltodextrins were less affected by the cross-linking process compared to low molecular weight maltodextrins and were better able to maintain their fibrous morphology. The resultant cross-linked maltodextrin fibrous membrane still contains some water soluble low molecular weight fractions which are removed by washing of the cross-linked fibers. Cross-linked higher molecular weight maltodextrin fibers were able to retain more mass after the washing process with less than 13 wt% loss and with 33.3 wt% citric acid added prior to heat treatment.
Gelatin is a natural protein that exhibits good biocompatibility, biodegradability and is readily available commercially. This protein may be dissolved in water at elevated temperature for electrospinning but its water solubility limits its applications. Etxabide et al (2022) showed that it is possible to blend cross-linking agents into the gelatin solution to render the electrospun membrane insoluble in water. Using the Maillard reaction (MR), a condensation reaction between proteins and sugar, Etxabide et al (2022) blended ribose (a sugar) into the gelatin solution prior to electrospinning. With the addition of ribose, the resultant gelatin fibers were insoluble in water although the fibrous morphology was compromised when the electrospun membrane was soaked in water at 37 °C. Heat treatment of the ribose/gelatin fibers induces cross-linking and with heat treatment temperature of 110 °C and a ribose concentration of 20 wt% the electrospun ribose/gelatin membrane was able to retain the fibrous morphology after soaking in 37 °C water for 24 h although fusion of fibers still occurred.
Certain natural solvents may be used to dissolve industrial polymers for electrospinning. D-Limonene is a natural solvent made of monoterpene hydrocarbon and is the main component of orange or lemon peel oil. Khirandish et al (2016) demonstrated the use of this solvent in the electrospinning of polystyrene (PS) containing titanium dioxide (TiO2 nanoparticles. To facilitate the electrospinning, the solution containing PS and TiO2 nanoparticles were spike with LiCl salt. Using this solution, they were able to generate smooth PS nanocomposite fibers with diameters ranging from 1.7 to 2.1 µm.
Apart from natural occurring solvents, researchers have also explored the use of "green" solvents which are solvents produced from biomass. For electrospinning of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), Lapomarda et al (2022) dissolved the polymer in solvents derived from levulinic acid (LA, 4-oxopentanoic acid) which itself was produced from both hemicellulosic and cellulosic fractions of biomass. From the selection of solvents, it was found that a combination of 50% v/v formic acid and 50% v/v methyl ethyl ketone (MEK) was able to dissolve PHBV and produce smooth fibers through electrospinning. However, the mixture of solvents and PHBV needs to be heated to 60 °C for complete dissolution and during the electrospinning process, the solution was maintained at the same 60 °C to ensure sufficient evaporation of the solvents upon fiber deposition so that distinct fibers can be collected.
A whole class of material has been made into nanofibers using non-toxic solvents and polymers as binder or carrier. A wide variety of inorganic nanofibers have been fabricated by electrospinning its precursors and blended with water-soluble polymers. The polymers are usually removed during the sintering process. The resultant inorganic nanofibers has been tested for use in diverse applications such as catalyst, sensors, electronic devices, batteries and energy generation.
For some polymers, a less toxic solvent may be used for its dissolution and electrospinning. Poly(2-n-propyl-2-oxazoline) (PnPrOx) is a polymer that exhibits lower critical solution temperature (LCST) behavior where it becomes water soluble below its LCST (~25 °C) and insoluble above it. However, LCST behavior of PnPrOx in water is not observed at high concentration required for electrospinning as the polymer would be insoluble. Addition of ethanol into the water allows dissolution of higher PnPrOx concentration for the purpose of electrospinning. PnPrOx nanofibers were able to exhibit LCST behavior with complete dissolution below the determined cloud point temperature values of about 19 °C - 23 °C. Cross-linking such as using tannic acid is able to protect the fibers from dissolving in water.
Zein is a natural polymer derived from corn grains. However, its poor mechanical strength meant limited usage in its pure form. Jia et al (2025) used a core-shell fiber structure with a blend of zein and shellac to improve the mechanical properties of the resultant electrospun membrane. Shellac is a natural amphiphilic biopolymer derived from the female lac insect. In the preparation of the core-shell electrospun fibers, the core was a blend of shellac and zein in ethanol aqueous solution and the shell was polyvinylpyrrolidone (PVP) in ethanol aqueous solution. At optimal shellac to zein ratio, the mechanical strength increased from pure zein at 0.57 MPa to 1.15 MPa. The significant increase in mechanical strength was attributed to strong hydrophobic interactions and hydrogen bonding between zein and shellac molecules.
Wei et al (2025) constructed a crosslinked electrospun zein/polyethylene glycol (PEG)/nisin membrane for the purpose of food packaging. Both zein and PEG were used as the matrix material with PEG giving strength to the material while nisin is an antibacterial compound. All three materials were dissolved in ethanol for electrospinning into nanofibrous membranes. The electrospun membrane was crosslinked using citric acid vapor by placing citric acid solution and the membrane in a chamber heater at 60 °C for 30 min. Citric acid was used as it is generally regarded as a safe crosslinked. The crosslinked membrane was found to be effective in inhibiting Staphylococcus aureus and Escherichia coli.
Perhaps the most benign form of electrospinning is to avoid the use of solvent altogether. Melt electrospinning uses heat to melt the polymer for electrospinning. This process does not require any solvent and is probably the cleanest form of fiber production. However, its main challenge is to bring the fiber diameter to less than a micron. Using solution-based electrospinning, fibers in the hundreds of nanometer can be quite easily produced. Melt electrospinning in contrast frequently spun fibers in the microns and tens of microns diameters. Careful optimization process is needed to bring it to the low microns diameter and there are some reported successes in getting fiber diameter below that [Bubakir et al 2014].
To improve the environmental sustainability of melt electrospinning, naturally derived polymers and additives may be selected for production of fibers. Koenig et al (2020) examined the use of biobased dyes, alizarin, hematoxylin and quercetin as conductive additives to reduce the diameter of polylactic acid (PLA) fibers produced by melt electrospinning. All three biobased additives were able to reduce fiber diameter compared to pure melt electrospun PLA. The three additives had different influences on PLA properties and hence the mechanism of diameter reduction. The greatest diameter reduction was produced using 2% (w/w) hematoxylin which reduced the average fiber diameter by 77% with a fiber diameter of 16 µm. It was found that at the melt spinning temperature of 275 °C, there is degradation of the polymer with hematoxylin and the reduced molecular weight favoured the reduction in fiber diameter. For alizarin, its addition increases the viscosity of the PLA melt and a plasticizer was added to mitigate it. Alizarin increases the conductivity of the PLA and the resultant combination produces a diameter reduction to 24 µm, a 63% diameter reduction. Finally, with the addition of quercetin, it reduces the melt viscosity but has limited influence on the electrical conductivity. A melt fiber diameter of 37 µm was collected.
Water based electrospinning may also be used to produce membranes with antibacterial properties. Moganti et al (2016) used a mixture of chitin, bio-lignin nanoparticles and polyethylene oxide to form a gel with water for electrospinning. Chitin is known to exhibit antibacterial properties and separate tests of the chitin added to polysulfonic membrane demonstrated antibacterial activity. Further modifications of the electrospun chitin-biolignin-PEO are needed to obtain insoluble membrane form for filtration and other applications.
Published date: 08 November 2016
Last updated: 02 December 2025
▼ Reference
-
Bubakir M M, Li H, Wu W, Li X, Ma S, Yang W. Applications of web produced by hot air assisted melt differential electrospinning method. IOP Conf. Series: Materials Science and Engineering 2014; 64: 012052.
Open Access
-
Cecone C, Hoti G, Zanetti M, Trotta F, Bracco P. Sustainable production of curable maltodextrin-based electrospun microfibers. RSC Adv. 2022; 12: 762.
Open Access
-
Etxabide A, Akbarinejad A, Chan E W C, Guerrero P, Caba K, Travas-Sejdic J, Kilmartin P A. Effect of gelatin concentration, ribose and glycerol additions on the electrospinning process and physicochemical properties of gelatin nanofibers European Polymer Journal 2022; 180: 111597.
Open Access
-
Faria J, Echeverria C, Borges J P, Godinho M H, Soares P I P. Towards the development of multifunctional hybrid fibrillary gels: production and optimization by colloidal electrospinning. RSC Adv. 201; 7: 48972.
Open Access
-
Gonzalez E, Barquero A, Muñoz-Sanchez B, Paulis M, Leiza JR. Green Electrospinning of Polymer Latexes: A Systematic Study of the Effect of Latex Properties on Fiber Morphology. Nanomaterials. 2021; 11(3):706.
Open Access
-
González E, Barquero A, Paulis M, Leiza J R. Fabrication of Multifunctional Composite Nanofibers by Green Electrospinning. Macromolecular materials & Engineering 2023; 308: 2300011
Open Access
-
Jia Q, Wu Z, Wang L, Zhang W, Li Y, Li S, Qin Y. Enhancing mechanical and thermal properties of zein films via shellac incorporation using coaxial electrospinning. LWT 2025; 219: 117556.
https://www.sciencedirect.com/science/article/pii/S0023643825002403Open Access
-
Khatri M, Khatri Z, El-Ghazali S, Hussain N, Qureshi U A, Kobayashi S, Ahmed F, Kim I S. Zein nanofibers via deep eutectic solvent electrospinning: tunable morphology with super hydrophilic properties. Sci Rep 2020; 10: 15307.
Open Access
-
Khirandish M, Borhani S, Mallakpour S, Youssefi M. Properties of PS/TiO2 electrospun fibres using limonene as a solvent. Indian Journal of Fibre & Textile Research 2016; 41: 373.
Open Access
-
Kianfar P, Bakry A, Dalle Vacche S, Bongiovanni R, Vitale A. Suspension electrospinning of SBR latex combined with photo-induced crosslinking: control of nanofiber composition, morphology, and properties. J Mater Sci 2024; 59: 3711.
Open Access
-
Koenig K, Balakrishnan N, Hermanns S, Langensiepen F, Seide G. Biobased Dyes as Conductive Additives to Reduce the Diameter of Polylactic Acid Fibers during Melt Electrospinning. Materials (Basel) 2020; 13: 1055.
Open Access
-
Komal R, Shoya T, Satoshi F. Biocompatible Native Hyaluronan Nanofibers Fabricated via Aqueous PEO-Assisted Electrospinning and Heat-Quench Process. ACS Omega 2024; 9:, 40010.
https://pubs.acs.org/doi/full/10.1021/acsomega.4c05851 Open Access
-
Kusumaatmaja A, Sukandaru B, Chotimah, Triyana K. Application of polyvinyl alcohol nanofiber membrane for smoke filtration. AIP Conference Proceedings 2016; 1755: 150006.
Open Access
-
Lapomarda A, Esposti M D, Micalizzi S, Fabbri P, Galletti A M R, Morselli D, Maria C. Valorization of a Levulinic Acid Platform through Electrospinning of Polyhydroxyalkanoate-Based Fibrous Membranes for In Vitro Modeling of Biological Barriers. ACS Applied Polymer Materials 2022; 4 (8): 5872.
Open Access
-
Lin S, Wang R, Yi Y, Wang Z, Hao L, Wu J, Hu G, He H. Facile and green fabrication of electrospun poly(vinyl alcohol) nanofibrous mats doped with narrowly dispersed silver nanoparticles. International Journal of Nanomedicine 2014; 9: 3937.
Open Access
-
Liu Z, Qin L, Liu S, Zhang J, Wu J, Liang X. Superhydrophobic and highly moisture-resistant PVA@EC composite membrane for air purification. RSC Adv., 2022; 12: 34921.
Open Access
-
Morganti P, Ciotto P D, Stoller M, Chianese A. Antibacterial and Anti-inflammatory Green Nanocomposites. Chemical Engineering Transactions 2016; 47: 201.
Open Access
-
Nagakawa Y, Kato M, Suye S, Fujita S. Fabrication of tough, anisotropic, chemical-crosslinker-free poly(vinyl alcohol) nanofibrous cryogels via electrospinning. RSC Adv. 2020; 10: 38045
Open Access
-
Schoolaert E, Cossu L, Becelaere J, Guyse J F R, Tigrine A, Vergaelen M, Hoogenboom R, Clerck K. Nanofibers with a tunable wettability by electrospinning and physical crosslinking of poly(2-n-propyl-2-oxazoline). Materials & Design 2020; 192: 108747.
Open Access
-
Seif S, Graef F, Gordon S, Windberg M. Monitoring Drug Release from Electrospun Fibers Using an In Situ Fiber-Optic System. Dissolution Technologies 2016; 23: 6.
Open Access
-
Stie M B, Kalouta K, Cunha C F B, Feroze H M, Vetri V, Foderà V. Sustainable strategies for waterborne electrospinning of biocompatible nanofibers based on soy protein isolate. Sustainable Materials and Technologies 2022; 34: e00519.
Open Access
-
Topuz F, Celebioglu A, Aytac Z, Uyar T. Influence of salt addition on polymer-free electrospinning of cyclodextrin nanofibers. Nano Ex 2020; 1: 020041.
Open Access
-
Wei L, Zhu S, Xiong G, Li J, Zhang W. Citric acid vapor-assisted crosslinking of zein/PEG composite nanofiber membrane embedded with nisin by electrospinning for the cooled goose meat preservation. Current Research in Food Science 2025; 10: 100983.
https://www.sciencedirect.com/science/article/pii/S2665927125000140 Open Access
-
Wu H, Kong D, Ruan Z, Hsu P C, Wang S, Yu Z. A transparent electrode based on a metal nanotrough network. Nature Nanotechnology 2013; 8: 421.
-
Zhang J F, Wang Y, Liao S, Lallier T, Wen Z T, Xu X. Photo-cross-linked Antibacterial Zein Nanofibers Fabricated by Reactive Electrospinning and its Effects against Streptococcus mutans. Oral Health and Dental Studies. 2017; 1:1.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech