Electrospinning is known to be a very versatile process for fabricating nanofibers. Since nanofibers have a very high surface area to volume ratio it is an attractive structure for numerous applications. Further, unlike nanoparticles, interlocking nanofibers from electrospinning means that it is usually not necessary to have a carrier to prevent dispersion. Since electrospinning requires a feed solution to have relatively high viscosity, polymers are the most commonly used material class for this process. However, with post-treatment, other material class such as metal oxide, carbon, metal and glasses may also be made into nanofibers with the help from electrospinning.
Polymer
Polymer is the most commonly used material class for electrospinning. A huge range of polymers have been electrospun which covers industrial polymers, biodegradable polymers, speciality polymers and natural polymers. Generally, these polymers should have a high molecular weight and can be dissolved in a solvent. For commercially important polymers such as polyethylene and polypropylene which dissolves in few solvents, melt electrospinning provides an alternative option. Other polymer such as polyaniline which has low molecular weight generally requires a second, electrospinnable sacrificial polymer to be blended with it for electrospinning.
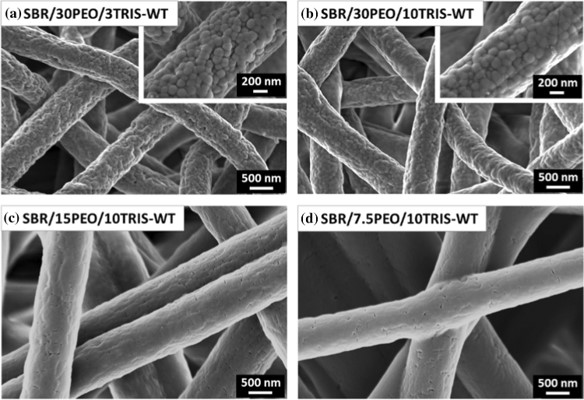
Latex beads may also be electrospun into fibers without dissolving in a solvent. Kianfar et al (2024) electrospun styrene-butadiene rubber (SBR) latex with photo-initiators and polyethylene oxide (PEO) as the electrospinnable binder. Post-electrospinning irradiation at ambient conditions were used to trigger the photo-induced thiol-ene crosslinking reaction. The PEO binder was subsequently washed off with water. Interestingly, despite the SBR latex beads having diameters in the range of 100 to 200 nm, and the electrospun fibers having diameters of 735 to 970 nm, the fibers showed a generally smooth surface both before and after the washing step although in higher PEO concentration SBR latex beads can be seen. With lower PEO concentration, the SBR latex beads were closer and this may encourage greater fusion during cross-linking hence giving a smoother fiber surface.
Short molecules and monomers
An essential requirement for electrospinning to form fiber is that the solution needs to withstand stretching without breaking up. Thus in general, higher molecular weight polymers are used for electrospinning as the chain entanglements allows it to be stretched without breaking. However, it is also possible to electrospin short molecules and monomers with sufficient intermolecular forces to hold the thread together during electrospinning. Locarno et al {2019} successfully electrospun simple amino acid, short peptide Fmoc-Phe-Gly fibers.The dipoles and Π-stacking of the aromatic systems hold the molecules together as it is being stretched under the strong electric field.
Topuz et al (2020) demonstrated the ability to electrospin hydroxypropyl-β-CD (HP-β-CD) and HP-γ-CD by dissolving them in water containing quaternary ammonium salt, tetraethylammonium bromide (TEAB), to increase the solution conductivity. Unlike polymers, CDs are oligomers and their solution viscosity does not come from chain entanglements. Electrospinning is possible due to hydrogen bond interactions among CD aggregates at high concentrations. Topuz et al (2020) was able to get beads free fibers at solution concentration of 180 w/v % and diameters less than 400 nm with 1 wt% (with respect to CD) TEAB in the solution. However, such small molecules are sensitive to additives that disrupt the hydrogen bonding which will cause beads formation or breaking up of the spinning jet into spraying instead.
Composite
Polymer matrix nanofiber composite can be routinely produced using electrospinning with electrospinnable polymers. Blending where fillers are mixed with the solution prior to electrospinning is the most common method of producing the nanofiber composite. Carbon nanotubes, clays, inorganic nanoparticles and many others have been routinely electrospun in a polymer carrier. Most reports of composite showed good distribution of the filler material below a threshold although surfactant may also be added into the solution to facilitate filler distribution.
Inorganic composite nanofiber may also be produced with electrospinning as the preliminary process. Inorganic nanofibers are commonly prepared by electrospinning of its precursor material followed by sintering process to remove the organic component. Inorganic composite fibers may be prepared by electrospinning a mixture of precursors and sintering.
Carbon
Since most polymers are made from a carbon backbone, carbon nanofibers can be made with post electrospinning carbonization process. Polyacrylonitrile (PAN) is one of the most commonly used polymer for conversion to carbon. PAN is also easily electrospun into nanofibers with N,N-dimethyl formamide (DMF) as the solvent. Other polymers have also been used for electrospinning and carbonization to yield carbon nanofibers [Nar et al 2016].
Metal oxide
Many metal oxides have important industrial applications due to their catalytic properties and photoactivity. Titanium dioxide is one of the most widely used metal oxides and can be found in solar cells, self cleaning surface and cosmetics. In the production of metal oxide nanofibers, the most common route would be to electrospin precursors either as a standalone solution or mixed with an electrospinnable polymer such as polyvinyl pyrrolidone (PVP). To produce titanium dioxide, titanium isopropoxide is often the precursor used. A post spinning sintering process is then used to convert the precursor nanofibers into the metal oxide nanofibers.
Metal
While bulk metals are often used as mechanical supports due to its high strength, at a much smaller dimension, metals are more commonly used as electrical conductors. The ability to produce metal nanofibers opens up a possibility of having nanowires on microelectronics. Currently, electrospinning is unable to produce metal nanowires directly but it can be part of a process to produce them.
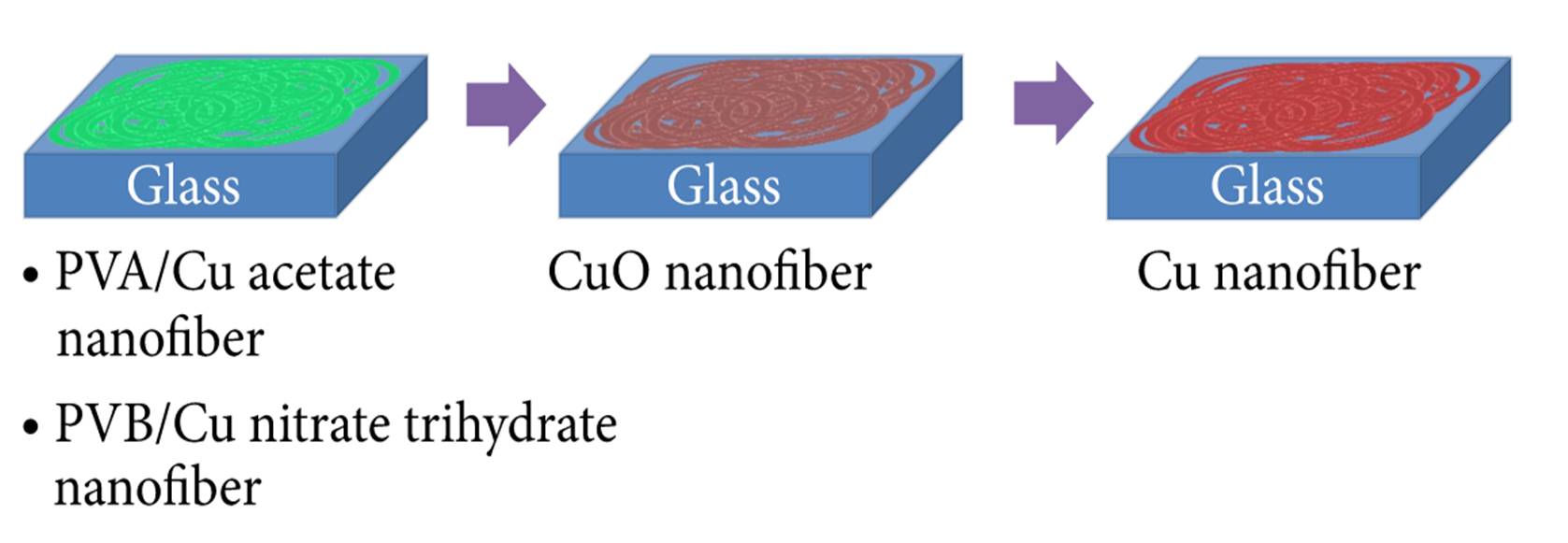
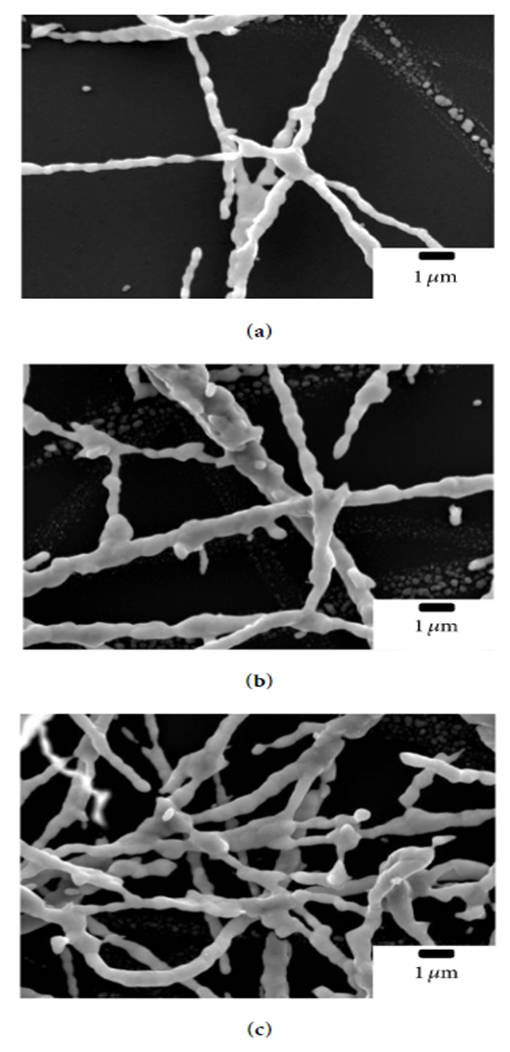
Just as metal oxide nanofiber can be fabricated from electrospun precursors and sintering, the metal oxide can be reduced to form metal nanofibers. This is demonstrated by Kim et al (2015) to fabricate copper (Cu) nanofibers. First, a mixture of polyvinyl butyral (PVB) and copper nitrate trihydrate was electrospun into nanofibers. The fibers were subsequently sintered to form copper oxide. The copper oxide nanofibers were then heated treated under hydrogen atmosphere to reduce it to copper nanofibers.
As shown in the SEM images below, the Cu nanofibers have a very uneven surface with several breaks between them. This could be due to the removal and reduction process which may cause migration and aggregation of molecules.
Another method that has successfully produced metal nanowire is to electrospin metal nanoparticles in a polymer carrier followed by sintering. Gries et al (2012) electrospun a solution containing a high concentration of gold nanoparticles to form nanofibers. Sintering was then carried out to remove the polymer matrix to leave behind a continuous gold nanowire.
Electrospun fibers may also be used as a template for metallic coating and subsequent sintering to form metal tubes. Sputtering where a metallic layer is coated on a surface can be used on electrospun nanofibers such that a sufficiently thick, self-standing layer is formed. Pantojas et al (2008) coated polyethylene oxide fibers with palladium using sputtering. A sputtering duration of more than 250s is required to form complete tubes after sintering. However, the thickness of the wall section of the cylinder is not uniform due to line-of-sight deposition.
Published date: 30 May 2017
Last updated: 21 January 2025
▼ Reference
-
Gries K, Vieker H, Gölzhäuser A, Agarwal S, Greiner A. Preparation of continuous gold nanowires by electrospinning of high-concentration aqueous dispersions of gold nanoparticles. Small 2012; 8: 1436.
-
Kianfar P, Bakry A, Dalle Vacche S, Bongiovanni R, Vitale A. Suspension electrospinning of SBR latex combined with photo-induced crosslinking: control of nanofiber composition, morphology, and properties. J Mater Sci 2024; 59: 3711.
Open Access
-
Kim S, Lee H, Kim D, Ko D A, Kim D, Kim H Yoon S G. Transparent Conductive Films of Copper Nanofiber Network Fabricated by Electrospinning. Journal of Nanomaterials 2015; 2015: 518589.
Open Access
-
Locarno S, Eleta-Lopez A, Lupo M G, Gelmi M L, Clerici F, Bitter A M. Electrospinning of pyrazole-isothiazole derivatives: nanofibers from small molecules. RSC Adv. 2019; 9: 20565.
Open Access
-
Nar M, Rizvi H R, Dixon R A, Kovalcik A, D'Souza N. Superior plant based carbon fibers from electrospun poly-(caffeyl alcohol) lignin. Carbon 2016; 103; 372.
-
Pantojas V M, Rodriguez D, Morell G, Rivera A, Ortiz C, Santiago-Aviles J J, Otano W. Synthesis of palladium with different nanoscale structures by sputtering deposition onto fiber templates. Journal of Nanophotonics 2008; 2: 021925.
-
Topuz F, Celebioglu A, Aytac Z, Uyar T. Influence of salt addition on polymer-free electrospinning of cyclodextrin nanofibers. Nano Ex 2020; 1: 020041.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech