Electrospinning has been used in the production of inorganic nanofibers by using its precursor solutions. To make the spinning process easier, polymers are often added to the solution which are removed following the sintering process. In some cases, while it is possible to electrospin inorganic precursors without the aid of a carrier polymer, having a carrier polymer is still preferred. Ramlow et al (2022) compared the resultant inorganic silicon carbonitride fibers from pyrolysis of electrospun polysilazane and polysilazane/polyacrylonitrile (PAN) fibers. The fibers from electrospun polysilazane/PAN have a smaller diameter than polysilazane. This may be due to the much lower concentration needed to obtain smooth electrospun fibers when PAN is added to the polysilazane solution. To get smooth electrospun fibers, pure polysilazane solution requires a concentration of 60 wt% while the mixture of polysilazane/PAN solution only requires 17.5 wt%. The presence of PAN may generate defects in the form of pores after pyrolysis although depending on the specific application, the pores may have the benefit of further increasing the surface area of the resultant fiber. The availability of precursors for many inorganic materials has resulted in the fabrication of a wide range of inorganic nanofibers using electrospinning. While there are different techniques for producing electrospun nanofibers such as needle-based electrospinning and needleless electrospinning, the mechanical performance of the resultant inorganic nanofibers are expected to be similar. This has been shown to be the case for electrospun TiO2 nanofibers [Vahtrus et al 2015]. However, given the greater stability of the solution jet for needle-based electrospinning, dimensional and mechanical consistency is better compared to needleless electrospinning [Vahtrus et al 2015].
Taking advantage of the high surface area of nanofibers and its form, new concepts and applications are been tested.
Most inorganic electrospun fibers are constructed from the sintering of its precursors. Certainly, sintering parameters such as temperature and heating rate will have an effect on the fiber form and material phase. Typically, the fibers undergo three phases during the sintering process. Phase one involves the removal of residual solvents and water vapors from the fibers. Second phase is where actual fiber shrinkage takes place as the organic materials are removed, polymerization, condensation and structural relaxation proceeds. Depending on the materials and temperature, phase three is where the inorganic material enters the glass transition stage [Shendokar et al 2008]. For production of inorganic fibers, most sintering ends at phase two. Preparation of most precursor solutions includes the addition of a carrier polymer, usually polyvinyl pyrrolidone or polyvinyl alcohol, to facilitate the formation of fibers during electrospinning. These polymers are typically soluble in water and ethanol, the same solvents used in dissolution of the salt for preparation of the precursor solution. These organic polymers will be removed during the sintering process. Jian et al (2022) showed the effect of sintering temperature on zinc oxide (ZnO) doped with 1.5 wt.% lanthanum (La) nanofibers. ZnO and La salt were blended with polyacrylonitrile (PAN) solution to form the precursor solution. With increasing sintering temperature, the ZnO crystallinity increases and this potentially reduces the recombination centers of photogenerated electrons and holes and hence increases photocatalytic efficiency. As the sintering temperature increases from 500 °C to 700 °C, the surfaces of the ZnO/La nanofibers become rougher as the PAN decomposes. However, at a sintering temperature of 700 °C, agglomeration of the oxide particles can be seen and this reduces the overall photocatalytic efficiency. Therefore, the highest photocatalytic efficiency was recorded for ZnO/La nanofibers sintered at 600 °C with 84% Rhodamine B decomposition in 510 min. There are some precursors that does not require a companion carrier polymer for electrospinning into fibers. Loccufier et al (2019) prepared an electrospinnable silica sol using tetraethyl orthosilicate (TEOS). To create the electrospinnable sol, a series of steps were needed to eventually form loosely cross-linked molecules to achieve the desired viscosity for electrospinning. However, even with optimized solution property, a stable electrospinning jet was only maintained for 2 to 5 minutes.
Since most precursors cannot be electrospun in its pure form, another method is to use coaxial electrospinning to construct a core-shell fiber with the precursor as the core and the carrier polymer as the shell. With polymer blended inorganic precursors, sintering and subsequent pyrolysis of the polymer will give rise to more porous fibers. However when the precursor is electrospun as a single component in the core of a core-shell fiber, reduced porosity, decreased surface defects, uniform crystal packing of the sintered fiber can be expected. Dong et al (2024) demonstrated this using polyvinylpyrrolidone (PVP) as the shell material and precursors of TiO2 and ZrO2 as the core. Polycrystalline TiO2 fibers after sintering and pyrolysis of PVP showed excellent flexibility and a high Young's modulus of 54.3 MPa. Similarly, ZrO2 fibers using the same process showed Young's modulus and toughness of 130.5 MPa and 11.9 KJ/m3, respectively. Both TiO2 and ZrO2 produced using the core-shell electrospinning method showed significantly better mechanical properties compared to the conventional blended method.
Electrospun inorganic nanofibers are generally made out of nano-size grains or crystals. The size of the grains would affect its specific surface area and this may in turn affect its ability to perform as sensors and catalyst. By varying the heating temperature, the grain size may be altered. Li et al (2012) showed that with TiO2, calcination of electrospun polyvinyl pyyrolidone/tetrabutyl titanate (PVP/TBT) at temperature of 500°C, 600°C and 700°C for 3 hours gave rise to increasing grain size. This results in a drop in its specific surface area which in turn reduces its photocatalytic activity. Chen et al (2019) constructed first transition-metal (Ti, Mn, Co, Ni and Zn) oxide nanofibers by electrospinning and tested their photocatalytic efficiencies. Ti, Mn, Co, Ni and Zn precursors were mixed with polyvinyl pyrrolidone (PVP) for electrospinning. Following calcination of the electrospun fibers, TiO2 and NiO nanofibers comprised of nanoparticles that were uniform and compact with an average diameter of 10 and 20 nm, respectively. However, the sizes of Mn2O3, Co3O4 and ZnO nanoparticles that form the fibers were relatively large, with sizes of 80-100, 60-80, 50-75 nm, respectively. They attributed these differences in the solubility of the respective salt in the PVP solutions. The precursors of TiO2 and NiO nanofibers were clear and transparent, while the other three precursors are homogeneous emulsions. During calcination, numerous nucleation sites were formed for TiO2 and NiO and these draw upon surrounding atoms to the crystal nucleus. For Mn2O3, Co3O4 and ZnO, particles were already present in the solution as evident by emulsion state. These act as ready nucleation sites during calcination and their rapid growth form larger nanoparticles. ZnO, TiO2 and NiO nanofibers exhibit excellent photocatalytic efficiency and high cycling ability to methylene blue (MB) under ultraviolet (UV) irradiation.
Inorganic crystals often form different phases depending on the molecular packing. For TiO2, rutile and anatase phases are the most commonly encountered and manufactured depending on the applications. The former is mainly for coating and cosmetics purpose due to its high refractive index and UV absorption cross-section and the latter is for catalysis as it is chemically and optically active. A simple method directing the phase formation and transformation is by controlling the calcination temperature. In electrospun TiO2, calcination temperature of less than 400°C produces mainly anatase phase while at temperature more than 600°C, rutile phase dominates [Song et al 2013].
Further study on the effect of annealing temperature on the TiO2 phases were carried out by Secundino-Sanchez et al (2019). In their annealed electrospun fibers, annealing temperature of less than 600°C gave pure anatase phase. At annealing temperature less than 800°C, mixed anatase and rutile phase was obtained and pure rutile phase was obtained at temperature between 800°C and 1000°C. During thermal treatment, lost of oxygen was evident and the vacancies due to this oxygen loss has been suggested to drive crystalline phase transformation. Radiative band red shift from 2.56 to 1.32 eV was recorded as the TiO2 nanofibers transformed from anatase to rutile phase with increasing treatment temperature.
The formation of crystals with specific facets orientation is influenced by parameters such as chemical used and pH of the precursors. Less known is the influence of the precursor processing method such as the use of electrospinning in controlling material phase and crystal facets orientations. Dai et al (2009) showed that with electrospinning, anatase TiO2 nanocrystals with exposed {001} facets can be fabricated. Electrospinning plays a critical role in this formation due to the small diameter of the nanofibers which allows moisture in the air to diffuse into the core for complete hydrolysis for Ti(OiPr)4 at a relative constant rate. Using other processing methods such as direct injection of the same solution into acid medium or forming cast films, the same crystals cannot be achieved.
Beyond crystal phase and orientation, the interaction of the precursor solution with the environment during electrospinning and subsequent calcination also lead to physical changes in the resultant fiber. From a single orifice nozzle, electrospun hollow inorganic nanofibers have been fabricated. In SnO2hollow fibers produced from electrospinning, it has been proposed that Kirkendall effect and surface diffusion during the calcination process has driven this formation [Xia et al 2012].
Electrospun inorganic fibers often demonstrate mesoporous structure as they are made from an assembly of nanocrystals. Titanium dioxide (TiO2) is commonly investigated for use as a photocatalytic agent and in solar cells applications. Thus various techniques have been designed to construct electrospun mesoporous TiO2 fibers. The simplest way of producing mesoporous TiO2 is by mixing TiO2 precursor such as titanium isopropoxide [Mondal et al 2014] or tetrabutyl titanate (TBT) [Li et al 2012] to a polymer material (polyvinylpyrrodidone) followed by electrospinning. Using a solution of V2O5 powder and poly(vinylpyrrodidone) (PVP), mesoporous vanadium pentoxide nanofibers has been fabricated after annealing at 500 °C in air for 1 h. The mesoporous fibers were found to exhibit excellent Li-ion storage capacity [Yu et al 2011].
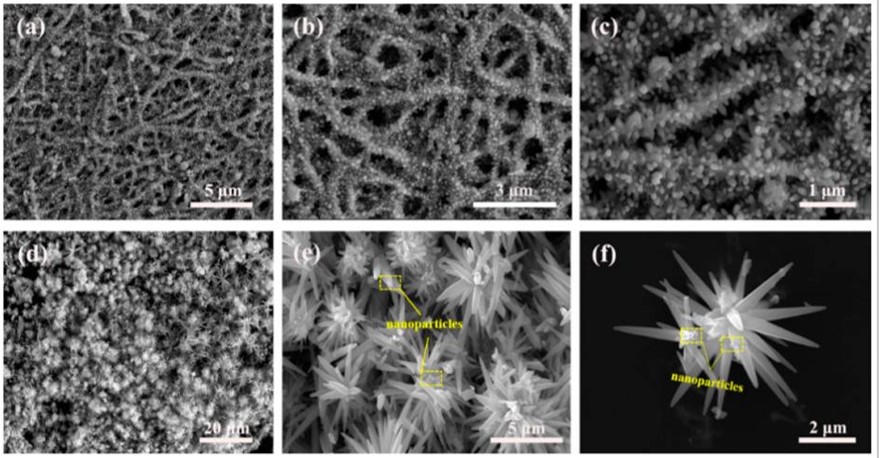
Fibers with surface projections may be constructed to increase surface area. Since inorganic fibers are used in many applications where higher surface area is desirable, researchers have shown the feasibility of using hydrothermal process to grow projections on the electrospun fibers. Fan et al (2019) produced electrospun ZnO nanofibers with two different type of surface projections. ZnO nanofibers were first produced by annealing of electrospun Zn(CH3COO)2·2H2O/polyvinylpyrrolidone (PVP) fibers. A reaction solution was prepared by mixing hexamethylenetetramine (HMTA) and zinc nitrate hexahydrate (Zn(NO3)2·6H2O) followed by the addition of ZnO. Hydrothermal reaction of this mixture produces fire cracker like surface. When the ZnO nanofibers were sonicated prior to hydrothermal treatment, flower like projections were produced.
Additives may be added to the precursor solution for electrospinning such that multiple reactions may take place during the annealing process to form hybrid structures. Wang et al (2019) constructed an electrospun membrane with dendritic hybrid architecture comprising of carbon nanotubes (CNTs) with nickel sulfide nanoparticles (NS) encapsulated inside, grown from the surface of porous electrospun N-doped carbon nanofibers (CNFs). The multi-stage process to construct this membrane starts with electrospinning of polyacrylonitrile (PAN) containing nickel acetate to form a fibrous membrane. During annealing, PAN was converted into porous N-doped carbon fibers which served as a reducing agent to convert Ni salt into Ni nanoparticles. Thiophene introduced during annealing, decomposes into H2S and gaseous hydrocarbons. The hydrocarbons served as the carbon source and the catalytic effect of Ni nanoparticles encouraged growth of carbon nanotubes (CNTs) epitaxially out of the CNFs. The Ni nanoparticles reacted with H2S to form nickel sulfides nanoparticles inside the CNTs. Pan et al (2019) fabricated CuCo2O4nanoparticles@N-carbon nanofiber (CuCo2O4NPs@N-CNFs) film by electrospinning of their precursors blend followed by carbonization/oxidation processes. The precursor solutions for forming carbon and CuCo2O4 was prepared separately. In this case, polyacrylonitrile (PAN) and polyvinylpyrrolidone (PVP) was used as the precursor for carbon and Cu(CH3COO)2 and 4.0 mmol Co(CH3COO)2 were the precursors for CuCo2O4.
These were then mixed together and stirred for a few hours before electrospinning. The resulting hybrid electrospun mat was carbonized and CuCo2O4 nanoparticles were formed during the same sintering process. The resultant CuCo2O4NPS@N-CNFs undergo a room-temperature in situ sulfurization by immersing into 2.0 m Na2S solution for a couple of hours. The CuCo2S4 NSs@N-CNFs) films showed remarkable bifunctional catalytic performance (Ej= 10 (OER) - E1/2 (ORR) = 0.751 V) with excellent mechanical flexibility.
Helical inorganic fibers have been constructed by sintering core-shell fibers with the pure inorganic precursors as the core. This was demonstrated by Dong et al (2024) using polyvinylpyrrolidone (PVP) as the shell material and precursors of TiO2 and ZrO2 as the core. The formation of the helical fiber has been attributed to crack formation on the surface of one side of the initial core-shell fiber during sintering. During electrospinning, stiffness of the core alkoxide sol as it vaporizes led to an uneven shrinkage of the ductile shell due to the constraint from the core-shell interface and the surface of the fiber. This resulted in cracks on the fiber shell, which distribute asymmetrically about the fiber axis and rotate along the change of the curving direction. As the fiber shrinks due to the surface PVP decomposition, the fiber starts to curl towards the side with the crack. With a thinner shell, the uneven shrinkage of the shell from the core-shell interface and its surface was less pronounced and no coiling of the fibers were observed.
Electrospinning has also been used to construct 3D nanofibrous inorganic structures. Dong et al (2023) showed that by having additives in the precursor solution to increase the conductivity of the solution was able to encourage the formation of in situ 3D structures from electrospinning. Using ethanol-based, titanium isopropoxide (TiP) and tetraethoxysilane (TEOS) solution with polyvinylpyrrolidone (PVP) and acetic acid (AcOH) for electrospinning, only 2D membranes were produced. By adding yttrium nitrate (Y(NO3)3·6H2O) to the solution, the electrospun jet forms a 3D structure. The addition of Y(NO3)3·6H2O increases the charge density and viscosity of the solution. Following calcination in air, TiO2/SiO2 ceramic fibers in both forms, 2D mats (TS-2D) and 3D assembly (TS-3D) were obtained. The highly porous 3D ceramic nanofibers assembly showed an ultralow density of about 3.6 mg/cm3 compared to a density of 18.8 mg/cm3 for its 2D form.
Sintering and annealing of electrospun inorganic precursors fibers are often conducted in a thermal convection furnace. This is usually a slow process as the heat is transferred from the heating element to the sample. Khishigbayar et al (2017) explored the use of microwave to convert electrospun polycarbosilane (PCS) to SiC fibers. Their result showed that the random and isotropic distribution of electrospun SiC fiber membrane was able to facilitate microwave adsorption. A temperature of over 800 °C was needed for conversion of PCS to SiC fibers. Using microwave radiation (2.45 GHz), temperature of 1000 °C was recorded on the electrospun PCS membrane. The temperature increases with higher membrane thickness due to greater microwave absorption. However, beyond a thickness of 1.44 mm, the radiation temperature remained constant at 1600 °C which represents a saturation of microwave absorption.
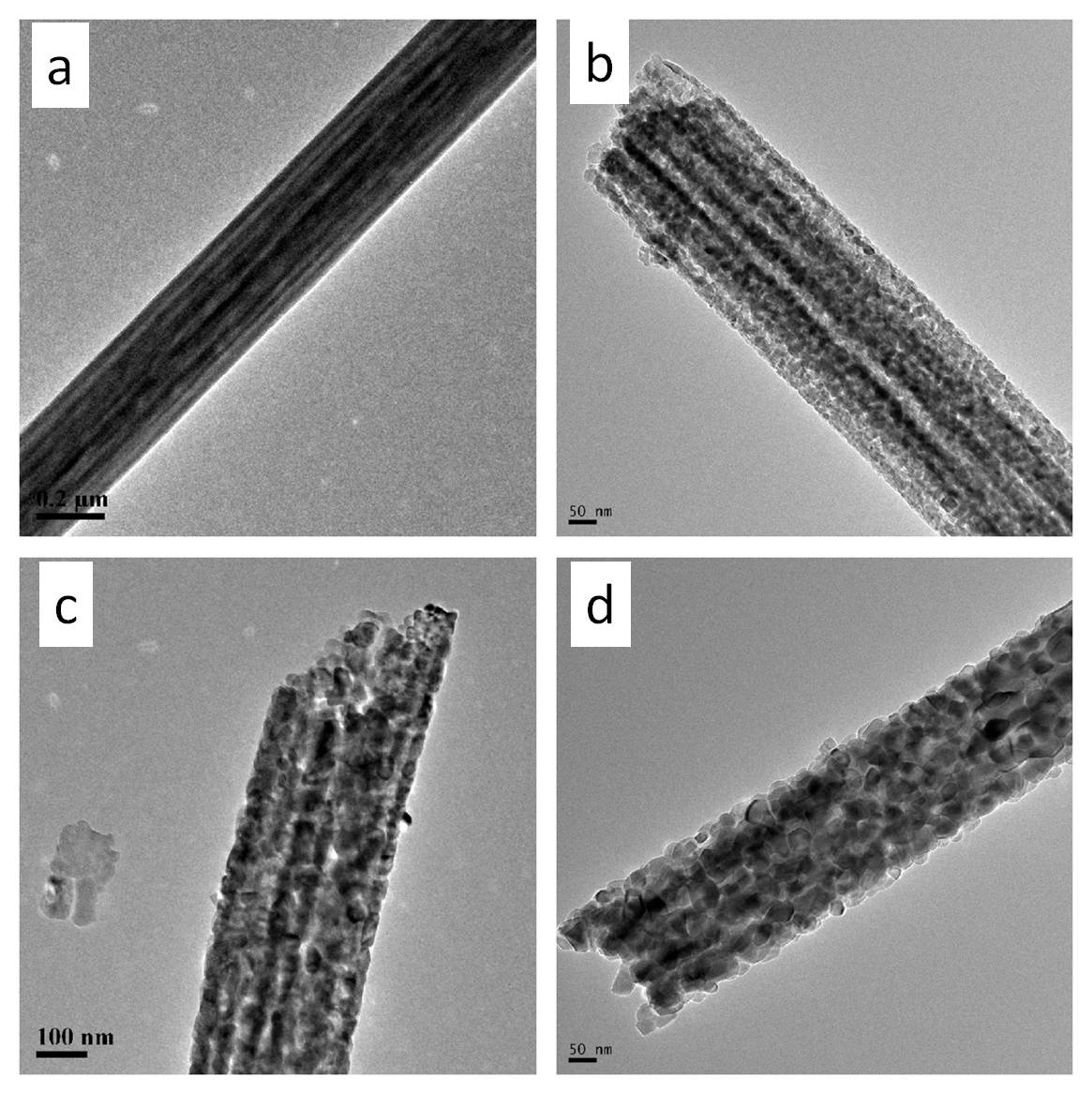
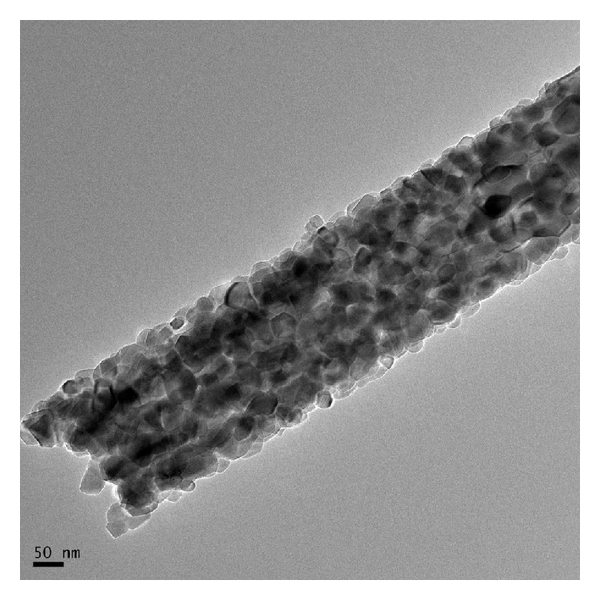
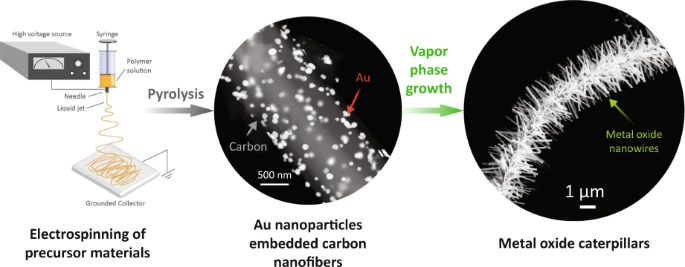
Electrospun fibers may be incorporated with catalytic additives for further processing. Vapor phase transport (VPT) uses catalysts embedded on a substrate to initiate and grow nanostructures off the substrate. Islam et al (2022) electrospin AuCl3/PAN nanofibres followed by carbonization to form Au nanoparticles (AuNPs)-decorated carbon nanofibres. The presence of AuNPs function as the catalyst for VPT growth in a furnace. A mixture of graphite powder and ZnO powder was used as the source for VPT growth in a furnace. The growth in the diameter and length of the nanostructures increases linearly with the furnace temperature. It is only at temperature of 1000 °C that microstructure starts to project from the nanofibers surface with gold nanoparticle droplets at the tip of many ZnO projections. In VPT, it is the metal vapor atoms that bind onto the catalyst. Therefore, higher temperature would increase the formation and movement of the metal vapor atoms and facilitate crystal growth. The same VPT condition yielded similar projections from In2O3 and SnO2.
Many inorganic materials are able to participate in electron transfer and this property has seen them used in many energy applications. Several electrospun inorganic nanofibers have been tested for use in dye-sensitized solar cell (DSSC). Perhaps the most frequently electrospun nanofiber for this application is based on TiO2. Unfortunately, energy conversion efficiency of electrospun TiO2 based DSSC is still relatively low at less than 7% [Fujihara et al 2007, Jin et al 2012]. Carbon nanofibers are commonly produced in laboratory using electrospun polyacrylonitrile (PAN) as its precursor. Electrospun carbon fibers have been investigated for use as electrode materials for energy storage [Mao et al 2013]. In lithium-ion batteries, electrospun nanofibers may be used as anode materials. To improve its performance, other electrochemically active metallic particles have been incorporated into electrospun carbon fibers.
Sensors are another application where inorganic electrospun fibers have found potential use. High surface area of nanofibers meant that the sensor would be very sensitive to stimulants. a humidity sensor [Khoshaman 2011]. Such sensors are very useful in high temperature environment due to its stability at those conditions [Ding et al 2010, see Introsensor.html]. For resistivity sensor, high surface area alone is not sufficient for its good performance. Fiber continuity is also important for transmission of electrons for faster response and recovery. Chen et al (2013) [see gasresistivitysensor.html] compared the performance of electrospun Co-doped SnO2 nanofibers and nanosphere for detection of methane. Although nanosphere has a larger surface area, nanofiber based sensor showed better gas response, higher saturated detection concentration, and quicker response-recovery time.
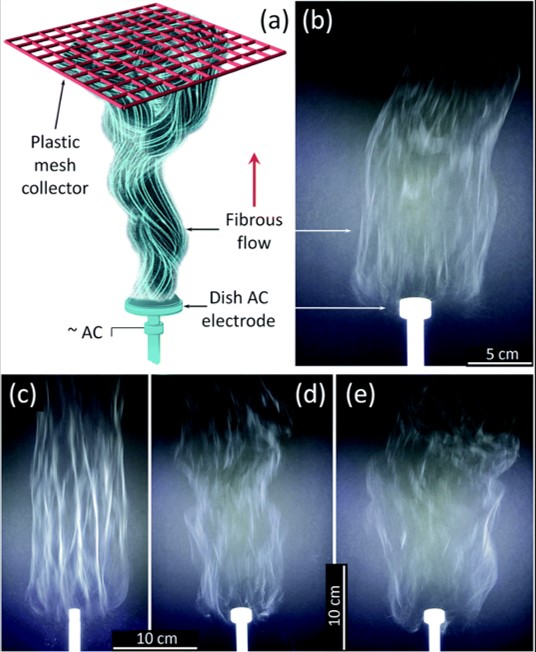
Inorganic nanofibers such as TiO2 require sintering of its electrospun precursor material. Since there will be further weight loss from the precursor material form, feasible commercial application of such inorganic nanofibers would require the development of mass production methods. Nealy et al (2020) used an alternating current (AC) on a free surface electrospinning setup to electrospin a solution of titanium(IV) n-butoxide (Ti(OBu)4)/ polyvinylpyrrolidone (PVP), with alternating current (AC)-voltages up to 40 kV rms at 60 Hz was used. The polymer solution was fed into a shallow dish-like electrode where the AC high voltage was applied. The collector was a PTFE plastic mesh placed about 50 cm above the electrode. Dish-like electrodes with diameters from 10 to 25 mm were tested. With smaller diameter electrodes, an increase of fiber bundling was observed which is not desirable. The feed-rate of the solution was adjusted to support a stable generation of fibers. Assuming the precursor fibers were converted to TiO2, the production rate of TiO2 nanofiber from one electrode is about 5.2 gh-1. A 5 - 7 cm thick fluffy layer of the fibers can be collected using this setup. The same setup has also been used to produce zirconium titanate nanofibers [Stanishevsky et al 2023].
Published date: 01 November 2016
Last updated: 14 October 2025
▼ Reference
-
Chen Y, Lu W, Guo Y, Zhu Y, Lu H, Song Y. Synthesis, Characterization and Photocatalytic Activity of Nanocrystalline First Transition-Metal (Ti, Mn, Co, Ni and Zn) Oxisde Nanofibers by Electrospinning. Appl. Sci. 2019; 9(1): 8.
Open Access
-
Dai Y, Cobley C M, Zeng J, Sun Y, Xia Y. Synthesis of Anatase TiO2 Nanocrystals with Exposed {001} Facets. Nano Letters 2009; 9: 2455.
-
Dong S, Maciejewska B M, Lißner M, Thomson D, Townsend D, Millar R, Petrinic N, Grobert N. Unveiling the Mechanism of the in Situ Formation of 3D Fiber Macroassemblies with Controlled Properties. ACS Nano 2023; 17: 6800.
Open Access
-
Dong S, Maciejewska B M, Schofield R M, Hawkins N, Siviour C R, Grobert N. Electrospinning Nonspinnable Sols to Ceramic Fibers and Springs. ACS Nano 2024; 18 (21): 13538.
https://pubs.acs.org/doi/full/10.1021/acsnano.3c12659Open Access
-
Islam M, Dolle C, Sadaf A, Weidler P G, Sharma B, Eggeler Y M, Mager D, Korvink J G. Electrospun carbon nanofibre-assisted patterning of metal oxide nanostructures. Microsyst Nanoeng 2022; 8: 71.
Open Access
-
Jian S, Tian Z, Hu J, Zhang K, Zhang L, Duan G, Yang W, Jiang S. Enhanced visible light photocatalytic efficiency of La-doped ZnO nanofibers via electrospinning-calcination technology. Advanced Powder Materials 2022; 1: 100004
Open Access
-
Jin E M, Zhao X G, Park J Y, Gu H B. Enhancement of the photoelectric performance of dye-sensitized solar cells using Ag-doped TiO2 nanofibers in a TiO2 film as electrode. Nanoscale Research Letters 2012; 7: 97.
Open Access
-
Fan C, Sun F, Wang X, Huang Z, Keshvardoostchokami M, Kumar P, Bo Liu B. Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties. Nanomaterials 2019; 9(9): 1277
Open Access
-
Fujihara K, Kumar A, Jose R, Ramakrishna S, Uchida S. Spray deposition of electrospun TiO2 nanorods for dye-sensitized solar cell. Nanotechnology 2007; 18: 365709.
-
Khishigbayar K E, Joo Y J, Cho K Y. Microwave-Assisted Heating of Electrospun SiC Fiber Mats. Journal of the Korean Ceramic Society 2017; 54: 499.
-
Li J, Qiao H, Du Y, Chen C, Li X, Cui J, Kumar D, Wei Q. Electrospinning Synthesis and Photocatalytic Activity of Mesoporous TiO2 Nanofibers. The Scientific World Journal 2012; 2012: 154939.
Open Access
-
Loccufier E, Geltmeyer J, Esquivel D, D'hooge D, D'Buysser K, D'Clerck K. Electrospinning of silica nanofibers without carrier polymer for advanced engineering applications. AUTEX2019, 2019.
Open Access
-
Mao X, Hatton T A, Rutledge G C. A Review of Electrospun Carbon Fibers as Electrode Materials for Energy Storage. Current Organic Chemistry 2013; 17: 1390.
-
Nealy S L, Severino C, Brayer W A, Stanishevsky A. Nanofibrous TiO2 produced using alternating field electrospinning of titanium alkoxide precursors: crystallization and phase development. RSC Adv., 2020; 10: 6840.
Open Access
-
Pan Z, Chen H, Yang J, Ma Y, Zhang Q, Kou Z, Ding X, Pang Y, Zhang L, Gu Q, Yan C, Wang J. CuCo2S4 Nanosheets@N-Doped Carbon Nanofibers by Sulfurization at Room Temperature as Bifunctional Electrocatalysts in Flexible Quasi-Solid-State Zn-Air Batteries. Advanced Science 2019; Article in press.
Open Access
-
Ramlow H, Marangoni C, Motz G, Machado R A F. Statistical optimization of polysilazane-derived ceramic: Electrospinning with and without organic polymer as a spinning aid for manufacturing thinner fibers. Chemical Engineering Journal Advances 2022; 9: 100220.
Open Access
-
Secundino-Sanchez O, Diaz-Reyes J, Sanchez-Ramirez J F, Jimenez-Perez J L. Structural and optical characterization of the crystalline phase transformation of electrospinning TIO2 nano?bres by high temperatures annealing. Revista Mexicana de Fisica 2019; 65 (5): 459.
-
Shendokar S, Kelkar A, Mohan R, Bolick R, Chandekar G. Effect of Sintering Temperature on Mechanical Properties of Electrospun Silica Nanofibers. Proceedings of IMECE2008 2008 ASME International Mechanical Engineering Congress and Exposition October 31-November 6, 2008, Boston, Massachusetts, USA
-
Song C G, Koppala S K, Yoon J W. Characterization of electrospun TiO2 nanofibers and its enhanced photocatalytic property under solar light irradiation. Journal of Ceramic Processing Research 2013; 14: 653. http://jcpr.kbs-lab.co.kr/file/JCPR_vol.14_2013/JCPR14-6/01.pdf
-
Stanishevsky A, Yager R, Nealy, S Severino C, Maniukiewicz W. High throughput fabrication of zirconium titanate nanofibers by using alternating field electrospinning. Materials Letters 2023; 330: 133318.
Open Access
-
Vahtrus M, Sutka A, Vlassov S, Sutka A, Polyakov B, Saar R, Dorogin L, Lohmus R. Mechanical characterization of TiO2 nanofibers produced by different electrospinning techniques. Materials Characterization 2015; 100: 98.
-
Wang A, Xie S, Zhang R, She Y, Chen C, Leung M K H, Niu C, Wang H. Chemical vapor deposition growth of carbon nanotube confined nickel sulfides from porous electrospun carbon nanofibers and their superior lithium storage properties. Nanoscale Advances 2019 Article in press .
Open Access
-
Xia X, Dong X J, Wei Q F, Cai Y B, Lu K Y. Formation mechanism of porous hollow SnO2 nanofibers prepared by one-step electrospinning. eXPRESS Polymer Letters 2012; 6: 169.
-
Yu D, Chen C, Xie S, Liu Y, Park K, Zhou X, Zhang Q, Li J, Cao G. Mesoporous vanadium pentoxide nanofibers with significantly enhanced Li-ion storage properties by electrospinning. Energy Environ Sci 2011; 4: 858.
▲ Close list
 ElectrospinTech
ElectrospinTech