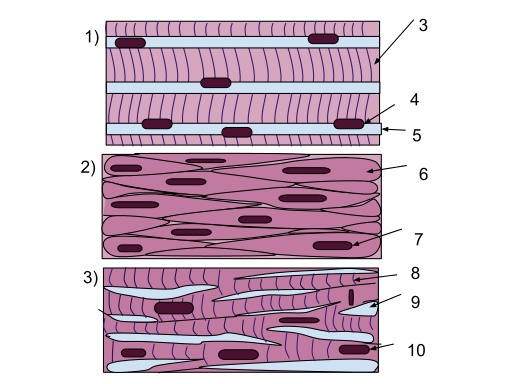
Muscle cells are generally organized in a specific direction for efficient contraction. There are three main types of muscle cells, the skeletal muscle, smooth muscle and cardiac muscle cells. Electrospun fibers have been tested as possible regenerative scaffolds for all three types of muscle cells. Scaffold properties such as material and fiber organization have been varied to test their effect on the muscle cells response.
The ability to electrospin scaffold comprising of aligned nanofibers make it attractive for regeneration of muscle tissue where contact guidance is able to encourage cell alignment. Such cell organization is induced without the need for chemical cues or fluid flow and has been demonstrated in different types of muscle cells. The potential of aligned electrospun fiber scaffold for skeletal muscle regeneration has been tested using C2C12 murine myoblasts by Aviss et al (2010). C2C12 murine myoblasts cultured on aligned poly-L-lactide-co-glycolide (PLGA) nanofibers with diameter of about 700 nm was able to demonstrate progressive alignment starting at 60 minutes after seeding with distinct alignment after 24 hours by tracking its cytoskeleton. In differentiation media, the cells showed distinct expression of fast myosin heavy chain with the fused multinucleated myotubes forming long myofibre-like cells aligned in the same direction. Such organization and level of fast myosin heavy chain expression were not observed in glass and randomly ordered nanofibers. Expression of sarcomeric myosin was also greater in the aligned fibers with the correct sarcometic protein array.
Electrical stimulation is another factor contributing to differentiation of C2C12 murine myoblasts to myotubes. Sirivisoot and Harrison (2011) seeded C2C12 murine myoblasts on electrospun polyurethane and polyurethane/carbon nanotubes mats and subjected them to electrical stimulation. Cells cultured on scaffolds with varying conductivity were subject to electrical stimulation. Greater number and length of myotubes were obtained for more conductive fibers as a result of carbon nanotube addition.
Native muscle tissues exist in three-dimensional blocks instead of thin sheet. To construct a block structure, Beachley et al (2014) seeded C2C12 myoblasts on sheets of aligned electrospun membrane made out of polycaprolactone (PCL) and stack them together with the help of fibrinogen and thrombin solutions. The polymerization of fibrinogen and thrombin formed fibrin which acts as glue between the cell sheets. C2C12 myoblasts were found to be aligned along the direction of the fibers.
A scaffold comprising of aligned fibers with adequate inter-fiber spacings will be a closer match to muscle ECM. However, aligned fibers will not be able to maintain an open structure without any support. Zhou et al (2018) used a trough collector comprising of a flat base and slanted walls to gather electrospun fibers along the depth of the collector. The collected fibers were aligned wall to wall to form a 3D structure supported by the walls. Fibroblasts cultured on this supported structure were found to be distributed throughout the fibrous matrix and aligned according to the fiber orientation. In this case, the supporting structure purpose is to hold the electrospun fibers in a 3D spatial form only while allowing the cultured cells to populate the scaffold. It will be interesting to determine the influence of such a aligned fibrous 3D scaffold on muscle cells.
Smooth muscle cells (SMC) are often found in blood vessels and digestive tracts where their contraction facilitates the movement of fluids in the lumen. Consideration for the material selection for them includes biocompatibility and mechanical properties. Hajiali et al (2011) tested the proliferation of human umbilical vein endothelial cells (HUVECs) on electrospun polyglycolic acid (PGA) scaffold with different ratio of gelatin added. HUVECs were found to proliferate best on PGA with 30wt% gelatin added after 5 days of culture. This is better than scaffold with lower gelatin concentration and much better than scaffold with 50wt% gelatin added. Better performance of PGA with 30wt% gelatin added may be due to optimum gelatin concentration or optimum fiber diameter which is about 517 nm. PGA with 10wt% gelatin added has smaller fiber diameter at 133 nm while with 50wt% gelatin added, the fiber diameter is 863 nm. Han et al (2019) investigated the effect of electrospun polycaprolactone (PCL) diameter on smooth muscle vascular cell (SMVC) infiltration and proliferation in In vitro tests and macrophage activation in a rat subcutaneous model. The tested fiber diameters range from 0.5 µm to 10 µm. Their results showed that smaller fiber diameter encourages greater cell proliferation with fiber diameters less than 1 & micro;m showing fastest proliferation and fiber diameters of 7 and 10 µm, the slowest. However, for cell Infiltration, fiber diameters of 5, 7 and 10 µm showed the best cell Infiltration. In terms of maintaining the SMVC contractile phenotype, there is a general reduction in the contractile phenotype as the fiber diameters increase. Fiber diameters less than 1 µm have the most number of cells maintaining the contractile phenotype at 10 days. In the In vivo studies larger fiber diameters increases the number of activated macrophages. The macrophages secrete angiogenic factors that stimulate neovascularization which may help to recruit sufficient my fibroblasts to populate the scaffold.
Cardiomyocytes are the muscle cells that provide the contraction force on the heart. Regeneration and replacement of the cardiomyocytes following myocardial infarction is vital to reduce the risk of future heart failure. Culturing of cardiomyocytes on electrospun poly(DL-lactide-co-glycolide) (PLGA) and poly(DL-lactide-co-glycolide)/gelatin (PLGA/Gel) membrane showed expression of cardiac specific proteins such as alpha-actinin and troponin I with better cell proliferation on PLGA/Gel membranes compared to PLGA [Prabhakaran et al 2011]. Zong et al (2005) showed that cardiomyocytes have a preference for relatively more hydrophobic surface such as poly(L-lactide) (PLLA) over PLLA/polyglycolide and poly(ethylene glycol) added. While the addition of gelatin reduces the hydrophobicity of the membrane, it is better able to encourage cell proliferation. Electrospun fibers may also be incorporated witih biomolecules and proteins to facilitate cell growth and proliferation. Electrospun polycaprolactone (PCL) coated with thymosin β4, a cardiomyocyte growth factor, was showed to exhibits much better cell growth and proliferation compared to PCL without coating [Kumar et al 2014]. Cardiomyocytes were also shown to be guided by underlying oriented nanofibers [Zong et al 2005].
Electrospun fibers have been widely investigated for use as a drug carrier. Therefore, it is able to function more than just a supporting structure for muscle regeneration. Yuan et al (2014) showed that by loading electrospun poly (L-Iactide) (PLLA) fibrous scaffold with ibuprofen (IBU) for release at the implantation site, it is able to inhibit inflammatory response and promote regeneration. This has been demonstrated on Sprague-Dawley (SD) rats musculus sacrospinalis model, where regeneration process started on the 7th day, more than a week earlier than PLLA and control groups. IBU/PLLA group was found to exhibit lower level of inflammatory factors and higher expression of repair factors. There are no statistical differences between the PLLA group and the control group in their healing process. Therefore, PLLA alone does not facilitate healing of the muscle lesions.
Published date: 28 April 2015
Last updated: 20 August 2019
▼ Reference
-
Aviss K J, Gough J E, Downes S. Aligned Electrospun Polymer Fibres for Skeletal Muscle Regeneration. European Cells and Materials 2010; 19: 193.
Open Access
-
Beachley V, Hepfer R G, Katsaneviakis E, Zhang N, Wen X. Precisely Assembled Nanofiber Arrays as a Platform to Engineer Aligned Cell Sheets for Biofabrication. Bioengineering 2014; 1: 114.
Open Access
-
Hajiali H, Shahgasempour S, Naimi-Jamal M R, Peirovi H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. International Journal of Nanomedicine 2011; 6: 2133.
Open Access
-
Han D G, Ahn C B, Lee, J H, Hwang Y, Kim J H, Park K Y, Lee J W, Son K H. Optimization of Electrospun Poly(caprolactone) Fiber Diameter for Vascular Scaffolds to Maximize Smooth Muscle Cell Infiltration and Phenotype Modulation. Polymers 2019; 11: 643.
Open Access
-
Kumar A, Patel A, Duvalsaint L, Desai M, Marks E D. Thymosin β4 coated nanofiber scaffolds for the repair of damaged cardiac tissue. Journal of Nanobiotechnology 2014; 12: 10.
Open Access
-
Prabhakaran M P, Kai D, Ghasemi-Mobarakeh L, Ramakrishna S. Electrospun biocomposite nanofibrous patch for cardiac tissue engineering. Biomed. Mater. 2011; 6: 055001.
-
Sirivisoot S, Harrison B. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. International Journal of Nanomedicine. 2011;6: 2483.
Open Access
-
Yuan Z, Zhao J, Yang Z, Li B, Yang H, W. Cui W, Zheng Q. Synergistic effect of regeneration and inflammation via Ibuprofen-loaded electrospun fibrous scaffolds for repairing skeletal muscle. European Journal of Inflammation 2014; 12: 41.
Open Access
-
Zhou Y, Hu Z, Du D, Tan G Z. The effects of collector geometry on the internal structure of the 3D nanofiber scaffold fabricated by divergent electrospinning. The International Journal of Advanced Manufacturing Technology 2018 Article in press.
-
Zong X, Bien H, Chung C Y, Yin L, Fang D, Hsiao B S, Chu B, Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 2005; 26: 5330
▲ Close list
 ElectrospinTech
ElectrospinTech