Photocatalytic materials have the potential to be used in various applications such as self-cleaning surface, water purification and toxin neutralization in defense applications. Metal oxides often exhibit this characteristic and mostly used in the form of nanoparticles. However, despite having the largest surface area to volume, they are susceptible to agglomeration which reduces its effective surface area. Further, the nanoparticles may need a carrier in actual field application and this will certainly reduces its effectiveness. As a porous nanofibrous network, the effective surface area can be maintained and with sufficient strength, the nanofiborus membrane may be used as it is.
In terms of photocatalytic materials, TiO2 is probably one of the most widely investigated. Checking the performance of the photocatalytic material is usually based on decomposition of selected test material such as methylene blue under light within a particular wavelength. Photocatalysis by TiO2 may be activated by ultraviolet (UV) light where electrons generation drives the photodegradation process. An alternative path using visible light is based on the dye absorbing the visible light and using TiO2 as a catalyst to bring the electron carriers and acceptors on the dye together [Jing et al 2012].
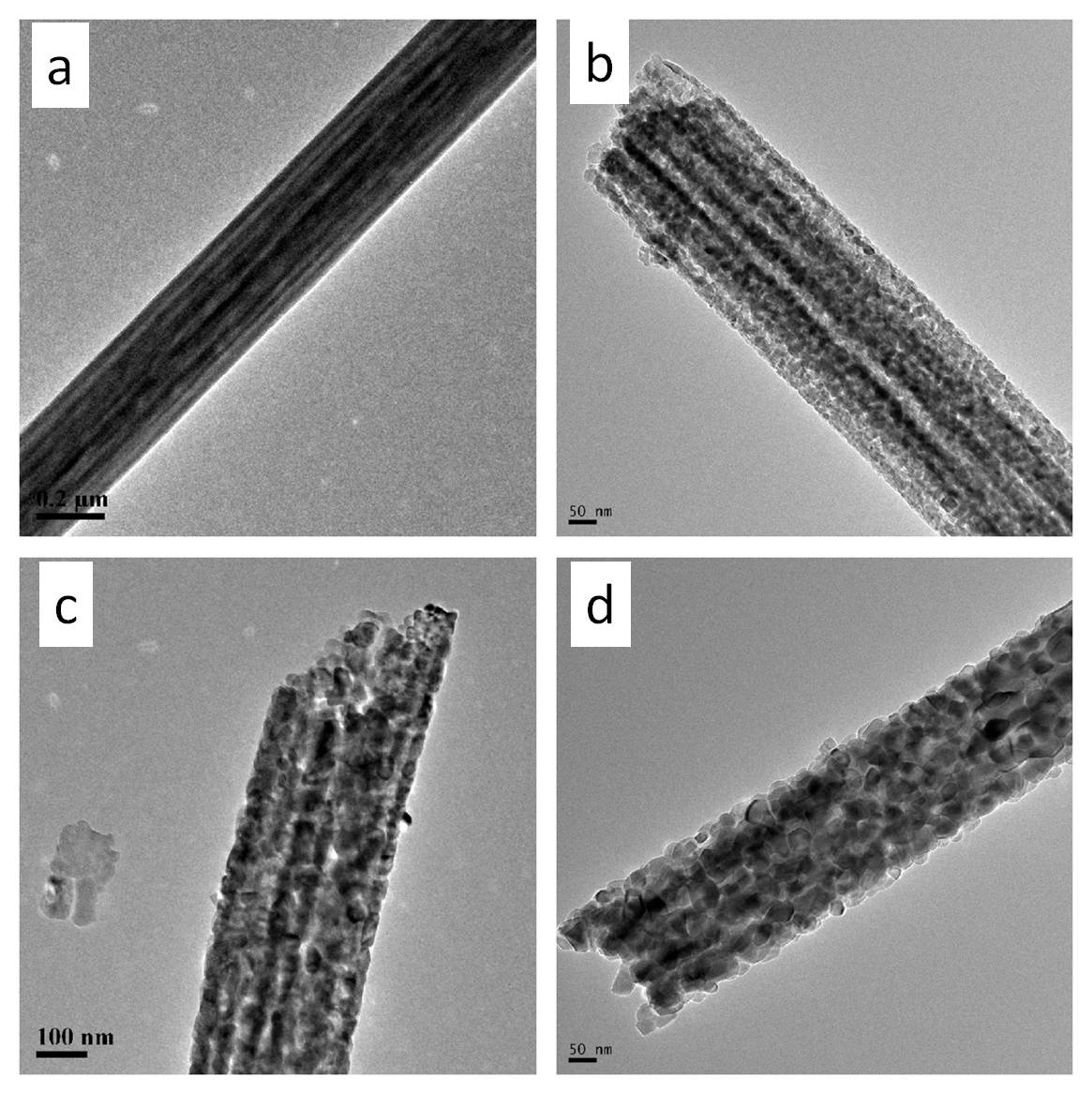
While it is widely accepted that the anatase phase is what gives TiO2 its photocatalytic property, Li et al (2022) suggest that a small amount of rutile phase in a majority anatase phase will give the electrospun TiO2 fiber the highest photocatalytic performance. In their research, the interfacial temperature between anatase and rutile phase occurs at 700 °C instead of 600 °C reported by others. While it is mainly anatase phase when annealed at 700 °C, a small amount of rutile phase can be found. Li et al (2022) hypothesized that the two phases in close proximity to each other forming an anatase-rutile heterojunction resulted in the highest photocatalytic action compared to pure anatase phase TiO2 from lower annealing temperature. Since anatase and rutile phase occupy different bands, the electron generated from the anatase conduction band (CB) would migrate to the rutile phase CB and the corresponding hole from the valence band (VB) of rutile phase to the VB of anatase phase. This improves the separation efficiency of the photogenerated electron-hole pair and reduces its recombination.
Chen et al (2019) constructed first transition-metal (Ti, Mn, Co, Ni and Zn) oxidse nanofibers by electrospinning and tested their photocatalytic efficiencies. Their result showed that ZnO, TiO2 and NiO nanofibers exhibit excellent photocatalytic efficiency and good cycling ability to methylene blue (MB) under ultraviolet (UV) irradiation while Mn2O3 and Co3O4 nanofibers could not induce the degradation of MB. Irradiation with UV light for 150 mins was able to induce 97.6%, 93.8% and 86.7% decomposition of MB using ZnO, TiO2 and NiO nanofibers respectively. The poor photocatalytic efficiency of Mn2O3 and Co3O4 nanofibers may be attributed to their larger particle size which may cause the electrons (e-) to recombine with holes (h+) in the process of reaching the conduction band before reaching the surface of the particles, Recycling the nanofiber membrane for the third round showed photocatalytic efficiency remained at 95.9%, 87.1% and 65.4% for ZnO, TiO2 and NiO nanofibers respectively. Of the first transition-metal (Ti, Mn, Co, Ni and Zn) oxidse nanofibers, ZnO demonstrated the best photocatalytic efficiency and cycling ability with TiO2 a close second.
The location of the photocatalytic material on the electrospun membrane may have a significant influence on its photocatalytic activity. AlAbduljabbar et al (2021) tested the efficacy of degrading methyl orange with TiO2 nanoparticles either adhered to the surface of electrospun polyacrylonitrile (PAN) fibers or blended within the PAN fibers. The electrospun PAN membrane with surface coated TiO2 nanoparticles was constructed by electrospraying a suspension of TiO2 nanoparticles over the prefabricated PAN nanofibers membrane. Comparing their photocatalytic activity, it was shown that PAN nanofibers membrane with surface coated TiO2 nanoparticles has a much higher activity at 92% against the TiO2/PAN blended sample at 49.6%. The difference in photocatalytic activity can be attributed to the greater exposure of surface TiO2 nanoparticles to methyl orange. Having TiO2 nanoparticles on the surface also increases the surface roughness and contributes to increased surface area of the membrane.
Where a polymer is used as a carrier for catalytic nanoparticles, the hydrophobicity of the carrier polymer needs to be considered especially when the nanoparticles are blended into the polymer matrix. Medeiros et al (2023) electrospun poly (butylene adipate-co-terephthalate) (PBAT) and poly (lactic acid) (PLA) fibers loaded with titanium dioxide-rutile (TiO2-R) and iron oxide-magnetite (Fe3O4) particles for pollutant photodegradation. With TiO2-R/Fe3O4 blended in PBAT/PLA, the onset of degradation only occurs after 300 min. This is probably due to the hydrophobicity of PBAT/PLA which delayed the contact between the model dye and the encapsulated photocatalytic particles. To mitigate this, the electrospun membrane was immersed in distilled water for 1080 min prior to testing. The immersed electrospun membrane showed a 20% improvement in photocatalytic efficiency compared to the membrane without immersion after 900 min.
In lab test between the photocatalytic performance between nanoparticles and nanofibers of the same material, the higher surface area of nanoparticles would give it an advantage [Liu et al 2012]. Tests by Li et al (2012) showed that smaller grain size nanofibers exhibits greater specific surface area and better self-photosensitization of Rhodamine (RhB). Since increasing the surface area of the nanofibers would enhance the performance of nanofibers this has led to several researches into introducing pores to the nanofibers. Liu et al (2012) mixed carbon nanosphere into TiO2 precursor solution before electrospinning. The resultant nanofibers which contained the nanospheres undergo calcination to form TiO2 nanofibers and during this process, the nanospheres were burnt off, leaving behind porous TiO2 nanofibers. Comparing the photocatalytic performance of porous TiO2 nanofibers, solid TiO2 nanofibers and TiO2 nanoparticles, porous TiO2 works the best but solid nanofibers showed the poorest result. Therefore, the better performance can be attributed to the high surface area of porous TiO2.
To enhance photocatalytic activity of inorganic material in visible light, dopants may be added to reduce the energy band gap. Samadi et al (2012) doped electrospun ZnO nanofibers with multi-walled carbon nanotube (MWCNT) and the doped ZnO demonstrated photocatalytic activity in visible light while pure ZnO nanofibers were inactive.
In the study by Jian et al (2022), ZnO from calcination of electrospun ZnO/PAN fibers showed a photocatalytic efficiency of 53.35% for Rhodamine (Rh B) at visible light wavelength. By doping ZnO nanofibers with 1.5 ?at.% lanthanum (La), the photocatalytic efficiency of Rh B was raised to 83.95% in 510 min of visible light illumination. The ZnO/La nanofibers were formed by blending precursors of ZnO and La in a polyacrylonitrile (PAN) solution followed by heat treatment. 1.5 ?at.% La was found to be the optimum amount for doping in the ZnO nanofibers to give the best photocatalytic efficiency. Higher concentration of La would cause a reduction in the photocatalytic efficiency which may be attributed to agglomeration La within the ZnO matrix. The addition of La reduces the band gap of ZnO due to free electrons from impurities leading to a new energy below the Fermi level and an additional electron energy level close to the conduction band. The reduction in band gap contributed to better utilization of photons which improves photocatalytic activity.
Other techniques such as nitridation of TiO2 have also been used successfully to enhance photocatalytic performance in visible light [Li et al 2012b].
Duan et al (2019) constructed a composite of TiO2 loaded with Au nanoparticles by electrospinning for photodegradation of rhodamine B (RB). Both TiO2 nanofibrous network and TiO2/Au nanofibers network was able to degrade almost 100% RB solution in 30min under UV irradiation, and TiO2/Au nanofibers exhibited faster degrading rate than pure TiO2 nanofibers. In visible light, pure TiO2 showed only a degradation rate of 5% in 120min. At optimum Au loading in TiO2, the degradation rate of RB was 42% in visible light. Under natural light, degradations of RB by using TiO2/Au nanofibers as photocatalysts reached almost 100% in 270min irradiation while the degradation by using pure TiO2 nanofibers was just 40%. The superior performance of TiO2/Au may be attributed to the plasmonic resonance absorption of Au nanoparticles and effective separation of photogenerated electrons and holes by the TiO2/Au heterojunction structures.
Wang et al (2021) constructed Fe-doped Nb2O5 nanofibers for visible light photocatalysis and was able to achieve a maximum degradation yield of 98.4% of Rhodamine B (RhB) under visible light irradiation for 150 min. The Fe-doped Nb2O5 nanofibers was constructed by electrospinning a mixture of NbCl5, FeCl3.6H2O and polyvinylpyrrolidone solution followed by annealing at 600°C. Under visible light, excited Nb2O5 generates electron-hole pairs but they readily recombine. With the introduction of Fe3+ ions, the Fe3+ ions were able to trap and transfer the electrons and holes which inhibits recombination and facilitates degradation reactions. Examining photoluminescence (PL) spectra showed that pure Nb2O5 nanofibers have higher recombination rate of electron-hole pair than that of 0.03FeNb nanofibers. The degradation rate of RhB remained above 94% with 6 cycles of photocatalytic runs which demonstrated the Fe-doped Nb2O5 nanofibers stability.
Wang et al (2021b) demonstrated the photocatalytic performance of graphitic carbon nitride (g-C3N4)/niobium pentoxide nanofibers (Nb2O5 nanofibers on photodegradation of rhodamine B and phenol under visible light irradiation. The structure of g-C3N4 is known to offer rapid photoinduced charge separation but its photocatalytic activity is reduced due to fast recombination of the photogenerated electron/hole pairs. The well matched band gap edges between Nb2O5 and g-C3N4 encourages charge carrier separation when used together. This significantly improves the photocatalytic performance over individual material. Nb2Cl5/ g-C3N4/PVP precursor solution was prepared and electrospun into nanofibers. Calcination of the precursor fibers gave nanofibers. UV-Vis absorption spectra results showed that g-C3N4 absorbs light in the visible region but pure Nb2O5 absorbs light in the UV spectra. The combination of Nb2O5/ g-C3N4 shifts the absorption edge into the visible light range. The band gap of the Nb2O5/ g-C3N4 is also narrower than pure Nb2O5 which favors photocatalytic activity in visible light. When the electrospun Nb2O5/ g-C3N4 nanofibers were tested with Rhodamine B (RhB), a photodegradation efficiency of 98.1% was recorded after 120 min of visible light irradiation and complete photodegradation of phenol over the same period . There were no apparent reduction in photocatalytic activity after four cycles of photodegradation and washing demonstrating the stability and reusability of the composite membrane.
Researchers have also tested nanofibers with different material organization to improve the photocatalytic performance. Zhong et al constructed flexible membranes of electrospun carbon nanofiber/tin(IV) sulfide (CNF/SnS2) core/sheath fibers for the purpose of wastewater treatment. SnS2 is able to generate photoelectrons under visible light irradiation due to its low band gap of 2.34 eV and this was shown to reduce water-soluble Cr(VI).
Maafa et al (2021) constructed a core-shell inorganic nanofiber by electrospinning a precursor solution of poly(vinyl alcohol), zinc acetate dihydrate, and cadmium acetate dihydrate followed by calcination. The resultant inorganic nanofiber was found to exhibit a Cd-rich core and Zn-rich shell. Such composite nanofiber has a much higher photocatalytic than pure ZnO nanofiber with methylene blue (MB) photo-degradation at 98% and 42% under 210 min of sunlight Irradiation for CdO/ZnO core/shell nanofibers and ZnO nanofibers, respectively. The better photocatalytic performance of CdO/Zn nanofibers over ZnO nanofibers may be attributed to lower band gap of CdO/Zn. With CdO/ZnO core/shell nanofibers, the generated electrons from irradiation of the surface ZnO were transferred to the conduction band of the CdO core. This reduces the recombination rate of the generated electron-hole pairs. The photo-generated holes and electrons would facilitate the formation of hydroxyl free radicals from water which oxidizes and degrades organic pollutants.
Electrospun inorganic fibers are generally brittle and may not be able to maintain its physical integrity during application. Another way of using electrospun fibers is to use it as a carrier for inorganic ions. He et al (2010) used electrospun fluoropolymer nanofiber mats with carboxyl groups for immobilization of TiO2-ZnS on its surface. The resultant TiO2-ZnS/fluoropolymer fiber composites demonstrated better degradation of methylene blue under visible light compared to TiO2 or TiO2-ZnS nanoparticles.
When a carrier is used for the photocatalytic material, factors such as the location of the photocatalytic material and the bonding between the photocatalytic material and carrier may have a strong influence on the photocatalytic activity. AlAbduljabbar et al (2021) used electrospun chitosan nanofibers as the carrier for TiO2 nanoparticles. Two methods of loading TiO2 nanoparticles were being investigated. The first method involves surface functionalization of chitosan nanofibers followed by electrospraying to deposit TiO2 nanoparticles on the surface of the nanofibers. In the second method, TiO2 nanoparticles were mixed into a chitosan solution and electrospun into fibers. Comparison of their photocatalytic performance was determined by the degradation of methylene blue dye. With the TiO2 nanoparticles on the surface of functionalized nanofibers, the photodegradation rate under UV light was 89.30% and a reaction rate constant k at 0.0088 min-1. For TiO2 nanoparticles that were blended into the chitosan nanofibers, the degradation efficiency under UV was only 40.26% and the reaction rate constant k was 0.0016 min-1. This significant difference in performance may be attributed to TiO2 nanoparticles on the surface of the fibers being more exposed to the UV light and in direct contact with the methylene blue molecules as compared to the blended system where the TiO2 nanoparticles were embedded within the carrier matrix material. The band gap of the TiO2 nanoparticles on the surface of functionalized nanofibers was also found to be smaller than the TiO2 nanoparticles/chitosan blended fibers. Surface roughness of chitosan nanofiber with TiO2 nanoparticles on its surface is also higher and this increases the exposed reaction site with the dye molecules.
In self-cleaning applications, the presence of photocatalytic materials will facilitate in the photo-degradation of surface stain. Zohoori et al (2014) loaded electrospun polyamide 66 nanofibers with nanoparticles (TiO2, SrTiO3 and ZnO) through solution blending. These fibers were heat-setted on surface of nylon fabric such that any stain on the electrospun composite fibers may undergo photocatalytic degradation. The layered fabric demonstrated self-cleaning property when stained with Direct Green 6 under UV irradiation. The fabric was able to maintain photo-activity after repeated laundering. Bedford et al (2010) constructed a self-cleaning textile fiber by using coaxial electrospinning. In their setup, the core of the fiber was made from cellulose acetate and the sheath was made from TiO2 nanoparticles. The core-shell fibers with TiO2 nanoparticles on the surface were able to fully degrade blue dye stains in 7 to 8 hours.
Electrospinning derived photocatalytic nanofibers have also been tested in the production of hydrogen from water splitting. Al-Enizi et al (2023) constructed ZnO/CdS nanofibers by electrospinning precursors of ZnO mixed with CdS nanoparticles (NPs). As ZnO is inactive in the visible light region, the addition of CdS which has a narrow-band gap was able to tune the ZnO band gap to harvest visible light. Production of H2 was carried out by placing the membrane in an aqueous solution containing Na2SO3/Na2S under light irradiation. Comparison was made with ZnO only nanofibrous membrane and CdS NPs. The ZnO/CdS composite nanofibrous membrane was able to produce up to 820 µmolh-1 g-1. This is much greater than ZnO nanofibrous membrane and CdS NPs which produced 115 µmolh-1 g-1, and 180 µmolh-1 g-1 respectively. Superior performance of the ZnO/CdS composite nanofibrous membrane was probably due to the high surface area of the nanofibrous membrane, absorption efficiency of visible light and the low photogenerated electron-hole recombination rate.
Published date: 03 January 2017
Last updated: 02 April 2024
▼ Reference
-
AlAbduljabbar FA, Haider S, Ali FAA, Alghyamah AA, Almasry WA, Patel R, Mujtaba IM. Efficient Photocatalytic Degradation of Organic Pollutant in Wastewater by Electrospun Functionally Modified Polyacrylonitrile Nanofibers Membrane Anchoring TiO2 Nanostructured. Membranes. 2021; 11(10):785.
Open Access
-
AlAbduljabbar F A, Haider S, Fekri Abdulraqeb Ahmed Ali F A A, Alghyamah A A, Almasry W A, Patel R, Mujtaba I M. TiO2 nanostructured coated functionally modified and composite electrospun chitosan nanofibers membrane for efficient photocatalytic degradation of organic pollutant in wastewater, Journal of Materials Research and Technology 2021; 15: 5197.
Open Access
-
Al-Enizi A M, Karim A, Yousef A. A novel method for fabrication of electrospun cadmium sulfide nanoparticles- decorated zinc oxide nanofibers as effective photocatalyst for water photosplitting. Alexandria Engineering Journal 2023; 65: 825.
Open Access
-
Bedford N M, Steckl A J. Photocatalytic Self Cleaning Textile Fibers by Coaxial Electrospinning. ACS Appl. Mater. Inerfaces 2010; 2: 2448.
-
Chen Y, Lu W, Guo Y, Zhu Y, Lu H, Song Y. Synthesis, Characterization and Photocatalytic Activity of Nanocrystalline First Transition-Metal (Ti, Mn, Co, Ni and Zn) Oxisde Nanofibers by Electrospinning. Appl. Sci. 2019; 9(1): 8.
Open Access
-
Duan Z, Huang Y, Zhang D, Chen S. Electrospinning Fabricating Au/TiO2 Network-like Nanofibers as Visible Light Activated Photocatalyst. Scientific Reports 2019; 9: 8008.
Open Access
-
He T, Zhou Z, Xu W, Cao Y, Shi Z, Pan W P. Visible-light photocatalytic activity of semiconductor composites supported by electrospun fiber. Composite Science and Technology 2010; 70: 1469.
-
Jian S, Tian Z, Hu J, Zhang K, Zhang L, Duan G, Yang W, Jiang S. Enhanced visible light photocatalytic efficiency of La-doped ZnO nanofibers via electrospinning-calcination technology. Advanced Powder Materials 2022; 1: 100004
Open Access
-
Li D, Xu K, Niu Z, Zhang C. Annealing and Plasma Effects on the Structural and Photocatalytic Properties of TiO2 Fibers Produced by Electrospinning. Catalysts. 2022; 12(11):1441.
Open Access
-
Li J, Qiao H, Du Y, Chen C, Li X, Cui J, Kumar D, Wei Q. Electrospinning Synthesis and Photocatalytic Activity of Mesoporous TiO2 Nanofibers. The Scientific World Journal 2012; 2012: 154939.
Open Access
-
Li H, Zhang W, Huang S, Pan W. Enhanced visible-light-driven photocatalysis of surface nitrided electrospun TiO2 nanofibers. Nanoscale 2012b; 4: 801.
-
Liu S, Liu B, Nakata K, Ochiai T, Murakami T, Fujishima A. Electrospinning Preparation and Photocatalytic Activity of Porous TiO2 Nanofibers. Journal of Nanomaterials 2012; 2012; 491927.
Open Access
-
Maafa I M, Al-Enizi A M, Abutaleb A, Zouli N I, Ubaidullah M, Shaikh S F, Al-Abdrabalnabi M A, Yousef A. One-pot preparation of CdO/ZnO core/shell nanofibers: An efficient photocatalyst. Alexandria Engineering 2021; 60: 1819.
Open Access
-
Medeiros AR, Lima FdS, Rosenberger AG, Dragunski DC, Muniz EC, Radovanovic E, Caetano J. Poly(butylene adipate-co-terephthalate)/Poly(lactic acid) Polymeric Blends Electrospun with TiO2-R/Fe3O4 for Pollutant Photodegradation. Polymers. 2023; 15(3):762
Open Access
-
Samadi M, Shivaee H A, Zanetti M, Pourjavadi A, Moshfegh A. Visible light photocatalytic activity of novel MWCNT-doped ZnO electrospun nanofibers. Journal of Molecular Catalysis A: Chemical 2012; 2012: 359.
-
Wang L, Li Y, Han P, Jiang Y. Facile fabrication of Fe-doped Nb2O5 nanofibers by an electrospinning process and their application in photocatalysis. RSC Adv., 2021; 11: 462.
Open Access
-
Wang L, Li Y, Han P. Electrospinning preparation of g-C3N4/Nb2O5 nanofibers heterojunction for enhanced photocatalytic degradation of organic pollutants in water. Sci Rep 2021b; 11: 22950.
Open Access
-
Zhong Y, Qiu X, Chen D, Li N, Xu Q, Li H, He J, Lu J. Flexible Electrospun Carbon Nanofiber/Tin(IV) Sulfide Core/Sheath Membranes for Photocatalytically Treating Chromium(VI)-Containing Wastewater. ACS Appl. Mater. Interfaces 2016; 8: 28671.
-
Zohoori S, Karimi L, Ayaziyazdi. A novel durable photoactive nylon fabric using electrospun nanofibers containing nanophotocatalysts. Journal of Industrial and Engineering Chemistry 2014; 20: 2934.
▲ Close list
 ElectrospinTech
ElectrospinTech