In plastic processing, additives are often added to the polymer to facilitate production or to impart certain properties or colourings to the final product. In electrospinning, salt may be added to a solution to influence its viscosity, surface tension and conductivity. Since solution conductivity has a major influence on the process and the influence of salt addition on this property will be covered in greater details.
Charges present in the solution has significant influence in the electrospinning process and the behaviour of the jet. To improve the conductivity of the solution, salt can be added so that they can function as carriers of the charges in the spinning solution and may play an important role in increasing the production rate of electrospinning. Salt may influence the electrospinning process in the following ways,
-
Increased stretching of the spinning jet (due to increased conductivity) therefore producing thinner fibers
-
More consistent distribution of charges leading to greater fiber uniformity
-
Reduce the surface tension of the solution
-
Reducing the incidence of beads formation
Electrospinning, stretching of the solution is influenced by the charges on the solution and the electric field. Without any additives to the solution to increase its conductivity or charge carriers, changes in the amount of charges on the solution and the strength electric field are controlled by the application of the high voltage to the spinneret. When the number of charge carriers in the solution is low, increasing the voltage applied only increases the electric field strength. Increasing electric field does not translate to greater stretching on the solution due to limited charges on the solution. To take advantage of the greater electric field strength due to increasing applied voltage, salt can be added to the solution to increase the number of charge carriers.
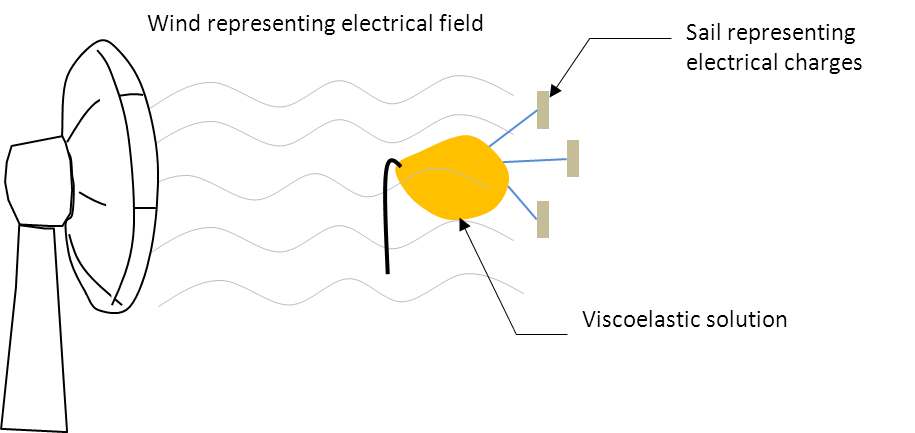
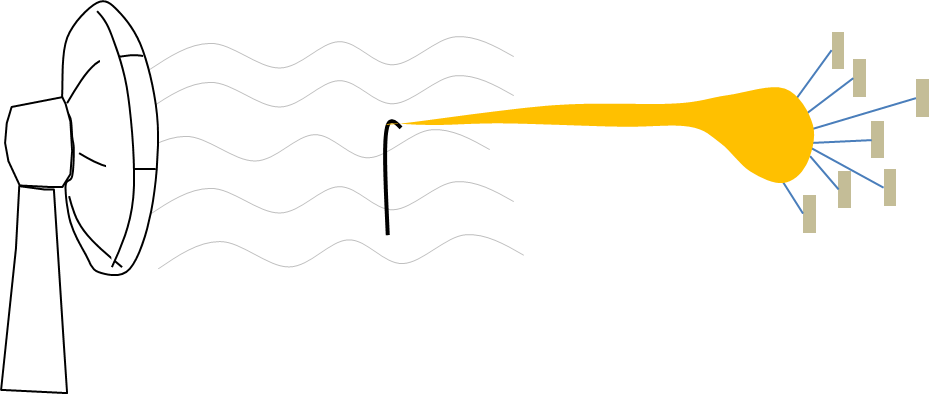
Illustration of charges and electric field on electrospinning solution
The relationship of charges and the electric field on the stretching of the solution can be illustrated by sail and wind with the sail representing the charges and the wind representing the electric field. With a viscoelastic solution attached to the sail, wind blowing against the sail will stretch the viscoelastic solution. Having more charges on the solution (such as having more charge carriers) means having more sails to catch the wind. Any slight increase in the wind will translate to a much greater stretching force. Conversely, when there are fewer sails, a stronger wind will only give a slight increase in the stretching force.
|
Stronger wind but less sails will result in small increase in solution stretching
|
|
More sails to catch the wind will result in greater solution stretching
|
|
Most studies showed that addition of small amount of salt will bring about a significant decrease in fiber diameter [Choi et al 2004] which is probably due to higher conductivity and corresponding stretching of the fiber. The same reason probably explain the spinning of smooth fibers when salt is added compared with beaded fibers from neat solution of the same concentration [Choi et al 2004, Arayanarakul et al 2006].
The use of salt to reduce fiber diameter in electrospun solution is probably more important in the electrospinning of small molecular weight molecules. For example, cyclodextrins (CDs) are oligomers and their solution viscosity does not come from chain entanglements. Electrospinning is possible due to hydrogen bond interactions among CD aggregates at high concentrations which would usually result in large fiber diameters. Topuz et al (2020) was able to get beads free fibers at solution concentration of 180 w/v % and diameters less than 400 nm with 1 wt% (with respect to CD) quaternary ammonium salt, tetraethylammonium bromide (TEAB) in the solution. While it is possible to electrospin beadless CD fibers without the addition of salt, the fiber diameter would be more than 1 micron. However, such small molecules are sensitive to additives that disrupt the hydrogen bonding which will cause beads formation or breaking up of the spinning jet into spraying instead.
Helgeson M E et al (2008) found that there is a significant decrease in the initial jet radius and a 3-fold increase in extension rate with the addition of NaCl to polyethylene oxide solution. The combination of an initial smaller radius and greater extension rate will favor smaller fiber diameter. However, there is a limit which the repulsive charges can exert versus the viscoelastic force of the solution and no further reduction of fiber diameter is possible with increasing salt content. Several authors reported an initial reduction in fiber diameter when NaCl is added but the trend reverses when more salt is added in their aqueous-based polymer solution [Arayanarakul et al 2006, Ding et al 2010]. Fiber quality may also deteriorate with more beads and greater variance in diameter beyond an optimum salt load [Ding et al 2010].
Such reversal in characteristic may be due to excessive charges on the solution leading to greater mass throughput [Arayanarakul et al 2006] or instability in the system [Ding et al 2010]. In a detailed study by Stanger J J (2008) using polyvinyl alcohol and various inorganic salts, he found that with the addition of salt, the deposition rate decrease, current flow increase and fiber diameter increases. This observation was attributed to higher charge density (with addition of salt) leading to a thinning in the initial jet radius and greater attraction to the collector. With reduction in the initial jet radius, less solution was drawn from the orifice and thus resulting in lower mass flow (deposition) rate. Higher charge density of the electrospinning jet and the subsequent larger attractive force towards the collector will encourage faster deposition of the fibers. As evident from the smaller deposition area, the electrospinning jet has less time to form larger loops and the shorter inflight duration will reduce the elongation of the jet resulting in larger fiber diameter. Optimal salt concentration in the solution is typically between 0.3 to 1 wt% of the solution [Arayanarakul et al 2006, Ding et al 2010].
Salt addition have also been shown to create other artefacts. While salt is typically added to increase solution conductivity for electrospinning, the salt may also alter other solution properties and this in turn affect the electrospun fiber. The addition of tetrabutylammonium chloride salt (TBAC) in polyamide-6 (PA6) solution for electrospinning resulted in chopped fibers [Ponce-Alcántara et al 2018]. It is unclear why this is so but the salt may have also alter the viscosity or surface tension. The addition of KCHOO to the solution initially reducs the electrospun PA6 fiber diameter but with more KCHOO added, the fiber diameter increases. It is only with pyridine salt that the solution surface tension and viscosity remain unchanged at low polymer concentration and concentration of pyridine increases to 5wt%. With this and 7wt% PA6 in the solution, the fiber diameter was brought down to 23 nm without any defects [Ponce-Alcántara et al 2018].
Instead of increasing the salt concentration to the solution, a study by Qin et al using different ionic salts and corresponding conductance in the solution showed that the diameter reduction in the fiber is proportional to increasing conductance of the solution [Qin et al 2007]. Therefore, the selection of salt with greater conductance may yield fiber of smaller diameter instead of reliance on the amount of salt added. While it has been suggested that ions with smaller atomic radius have a higher charge density and thus a higher mobility under an external electric field [Zong et al 2002], this has been disputed by Stanger J J (2008) who found little co-relation between the size of the salt and the fiber diameter. Instead, his study showed that it is the concentration of the ions and thus the conductivity of the solution rather than the ion size that influence the fiber diameter.
Apart from reduction in fiber diameter, addition salt has also contributed to some desirable quality in the process and the resultant fiber. Generally, the diameter distribution of electrospun fiber can be very wide but salt addition has been shown to significantly narrow this range [Lee et al 2005, Ding et al 2010]. At optimum salt content, the charges may be more uniformly distributed along the spinning jet and this may potentially facilitate uniform stretching of the fiber. Collection of the more highly charged spinning jet may be more efficient due to the jet's greater affinity towards a grounded target [Seo et al 2009, Yener et al 2011].
Although salt addition generally improves electrospinnability and reduces the fiber diameter, there are also exceptions where there is no significant change [Du et al 2008] or result in fiber of lesser quality [Lu et al 2008]. This is more evident in cases where there is strong polymer-solvent-salt interaction [Du et al 2008] or in hygroscopic salt [Yener et al 2011]. Li et al investigated the effect of salt addition on electrospinning of pullulan (PUL), a polysaccharide that may be used in food applications. Using NaCl and Na3C6H5O7, optimal concentration of the salts were 0.2M and 0.05M respectively. Less Na3C6H5O7 concentration required to produce fibers with similar diameter and quality to added NaCl may be due to the higher ionic strength of the salt. The presence of salt may interrupt the hydrogen bonding between the polymer chains and this may improve its solubility and increases solution viscosity as evident at low salt concentration. At higher salt concentration, the ions may interrupt hydrogen bonds between PUL and water molecules and this may result in a drop in solution viscosity. Increasing PUL fiber diameter at salt concentration above the optimum amount may be due to this effect. Salt crystals can be observed on the surface of the fibers when excessive salt was added to the solution.
Just as the addition of salt to solution electrospinning generally results in lower fiber diameter, reduction in the diameter of melt electrospun fibers can be achieved by incorporating polar additives such as stearic acid and sodium stearate. Chen et al (2014) demonstrated this effect by electrospinning polypropylene (PP) mixed with either stearic acid or sodium stearate by compounding. With 8 to 10 wt% of the additives, the electrospun PP fiber diameter was reduced from more than 5 µm to less than 2 µm. However, higher concentration of the additives reverses the trend leading to larger fiber diameter. Polar additives are heavily influenced by the electric field and this generates a greater stretching force on the electrospinning molten polymer which reduces the fiber diameter. However, at higher concentration of additives, agglomeration may takes place resulting in less uniform distribution and lower stretching force across the electrospinning jet length which leads to larger fiber diameter.
Published date: 08 Aug 2013
Last updated: 09 March 2021
▼ Reference
-
Arayanarakul K, Choktaweesap N, Aht-ong D, Meechaisue C, Supaphol P. Effects of Poly(ethylene glycol), Inorganic Salt, Sodium Dodecyl Sulfate, and Solvent System on Electrospinning of Poly(ethylene oxide). Macromol. Mater. Eng. 2006; 291: 581
-
Chen Z, He J, Zhao F, Liu Y, Liu Y, Yuan H. Effect of polar additives on melt electrospinning of non-polar polypropylene. J Serb Chem Soc 2014; 79: 587.
-
Choi J S, Lee S W, Jeong L, Bae S H, Min B C, Youk J H, Park W H. Effect of organosoluble salts on the nanofibrous structure of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Int J Biol Macromol. 2004; 34:249
-
Ding W, Wei S, Zhu J, Chen X, Rutman D, Guo Z. Manipulated Electrospun PVA Nanofibers with Inexpensive Salts. Macromol. Mater. Eng. 2010; 295: 958
-
Du J, Zhang X. Role of Polymer-Salt-Solvent Interactions in the Electrospinning of Polyacrylonitrile/Iron Acetylacetonate. J Appl Polym Sci 2008; 109: 2935
-
Helgeson M E, Grammatikos K N, Deitzel J M, Wagner N J. Theory and kinematic measurements of the mechanics of stable electrospun polymer jets. Polymer 2008; 49: 2924.
-
Li R, Tomasula P, Sousa A M M, Liu S C, Tunick M, Liu K, Liu L. Electrospinning Pullulan Fibers from Salt Solutions. Polymers 2017; 9(1): 32; doi:10.3390/polym9010032.
Open Access
-
Lee C K, Kim S I, Kim S J. The influence of added ionic salt on nanofiber uniformity for electrospinning of electrolyte polymer. Synthetic Metals 2005; 154: 209.
-
Lu X, Zhou J, Zhao Y, Qiu Y, Li J. Room Temperature Ionic Liquid Based Polystyrene Nanofibers with Superhydrophobicity and Conductivity Produced by Electrospinning. Chem. Mater. 2008; 20: 3420.
-
Ponce-Alcántara S, Martín-Sánchez D, Pérez-Márquez A, Maude J, Murillo N, García-Rupérez J. Optical sensors based on polymeric nanofibers layers created by electrospinning. Optical Materials Express 2018; 8: 3163.
Open Access
-
Qin X H, Yang E L. Wang S Y. Effect of Different Salts on Electrospinning of Polyacrylonitrile (PAN) Polymer Solution. Appl Polym Sci 2007; 103: 3865
-
Seo J M, Arumugam G K, Khan S, Heiden P A. Comparison of the Effects of an Ionic Liquid and
Triethylbenzylammonium Chloride on the Properties of Electrospun Fibers,1-Poly(lactic acid). Macromol. Mater. Eng. 2009; 294: 35
-
Stanger J J. Charge Transfer Mechanism in Electrospinning. MSc Thesis. University of Canterbury 2008.
Open Access
-
Topuz F, Celebioglu A, Aytac Z, Uyar T. Influence of salt addition on polymer-free electrospinning of cyclodextrin nanofibers. Nano Ex 2020; 1: 020041.
Open Access
-
Yener F, Jirsak O. Improving performance of polyvinyl butyral electrospinning. Nanocon 2011, 21. Czeh Republic, EU.
-
Zong X, Kim K, Fang D, Ran S, Hsiao B S, Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002; 43: 4403
▲ Close list
 ElectrospinTech
ElectrospinTech


