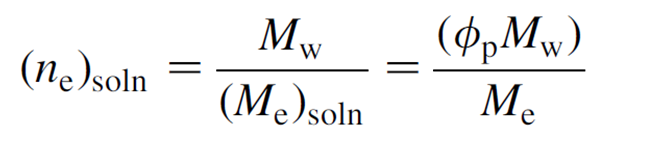The solution properties that significantly affect electrospinning are,
- Conductivity
- Viscosity
- Surface tension
- Solvent volatility
- Solution Phase Transitions (Fiber physical characteristics)
Conductivity and dielectric constant
When selecting the solvent(s) to dissolve the polymer, it is essential that one of the solvents have some degree of conductivity. Alternatively, organic or inorganic salt may be used to spike the solution. This also has the benefit of lowering the critical voltage required to initiate the electrospinning process [Son et al 2004]. However, this introduces impurity to the final polymeric product. Another useful method is to spike the solution with a mixture of solvent with one that is highly conductive.
Increasing the conductivity of the solution has been shown to improve the quality of the fibers. In cases where beaded fibers are formed, spiking the solution with salt has been shown to reduce or eliminate beads formation. This is due to increased stretching of the solution jet as a result of higher level of charges carried by the solution. The same factor also encourages the reduction of fiber diameter [Zhong et al 2002]. Another way to look at it is that increased bending instability due to greater conductivity lengthens the jet path as evident from the larger deposition area [Choi et al 2004].
Using near field electrospinning, Xue et al (2014) showed that as the conductivity increases, the deposited fibers transits from beaded to straight to curly. The transition from straight fibers to curly may be an evidence that the electrospinning jet speed increases with higher conductivity.
Another effect of increasing solution conductivity and dielectric constant is the number of electrospinning jets emitted from the tip of the spinning nozzle. Wu et al (2018) did an in-depth study on the dielectric value of the solution and its effect on multiple jets formation. Using a selection of solvents, they showed that as the dielectric constant of the solution increases, the number of jets increases. However, when the solution dielectric constant was reduced to single digit, there was only a single jet despite further increase in applied voltage. The stability of the jet may also be influenced by the solution dielectric constant. With a lower charge density, bending Instability may be reduced resulting in a longer stable electrospinning jet. The study by Wu et al (2018) using polystyrene (PS) in different solvents seem to support this although higher volatility of the solutions with higher dielectric constant used in their experiment may have also facilitated jet stability.
When it comes to precise stacking of fibers on top of one another as a form of precision electrospinning, a solution with high dielectric property may reduce the precision. Dielectric property of a material is its ability to store electrical energy. In near field electrospinning where the objective is to accurately position the deposited fibers, a solution of high dielectric constant and low solvent evaporation may increase charge retention and have a negative impact on fiber positioning. Sadaf et al (2022) used near field electrospinning to stack up polyethylene oxide (PEO) and they hypothesized that high dielectric constant of water led to high charge retention on the deposited fibers which disrupts the positioning of the fibers. To mitigate this, methanol which has lower dielectric constant, was added into the PEO aqueous solution. Another advantage of methanol is its faster evaporation and the resultant drier deposited fibers would have lower surface charge. However when the concentration of methanol to water exceeds 40%, stacking of the fibers deteriorates. This may be due to an overall reduction in the conductivity of the solution below an optimum level for stable deposition as methanol has a much lower conductivity than water. When water is replaced with dichloromethane (DCM) in the binary solvent system, a much higher layer of fibers can be stacked. DCM has a higher conductivity than water but a much lower dielectric constant than water and methanol. Using a DCM/methanol system effectively increases the conductivity and reduces the dielectric property of the solution. Further, DCM has higher solvent evaporation compared to methanol and this also helps to reduce the amount of residual charges on the deposited fibers. However, when the concentration of DCM to methanol exceeds 60%, rapid drying of the solution resulted in disrupted electrospinning. At optimum DCM/methanol ratio 6:4, a wall aspect ratio of 191.7?was achieved which is much greater than optimum water/methanol ratio 6:4 with wall aspect ratio of 80.
When selecting the type of salt to be added, there are several factors to consider. The effectiveness of the salt in reducing fiber diameter has been linked to its moleular weight. Salt with smaller molecular size was said to be more effective probably due to greater ion mobility as a result of its smaller size [Zhong et al 2002]. However, this has been disputed by the study from Stanger J J (2008) who found little co-relation between the size of the salt and the fiber diameter. Instead, his study showed that it is the concentration of the ions and thus the conductivity of the solution rather than the ion size that influence the fiber diameter. Salt such as triethyl benzyl ammonium chloride is able to increase the conductivity of the solution while reducing its surface tension [Lin et al 2004]. In some instances, the salt may interact with the polymer solution such that the viscosity increases. This may increase the diameter of the fibers instead if the repulsive force of the increased charges is less than the visco-elastic force [Mit-uppatham et al 2004].
While spiking the solution with more conductive solvents or additives is able to increase the solution conductivity, another method of increasing the solution conductivity is to use plasma treatment of the solution prior to electrospinning. Since electrospinning is strongly affected by molecular chain mobility and the conductivity of the solution, plasma treatment has the advantage of increasing solution conductivity as it ionizes both the polymer chain and solvent molecules. Viscosity of the solution may also increase due to greater interaction between the charged solvent molecules and polymer chains. Shi et al (2010) used atmospheric plasma treatment of polyethylene oxide (PEO) solution to demonstrate the influence on its electrospinnability. Their result showed enhanced conductivity, increased viscosity and surface tension following plasma treatment. The treated solution maintained a higher conductivity over untreated solutions over 240 min although it dropped significantly during the first 120 mins. In their experiment, electrospinning of the solution was carried out immediately after treatment. Electrospinning of treated solution was found to exhibit fewer beads and better quality fibers. Similar increase in solution conductivity for electrospinning following plasma treatment has been reported for other polymers such as polylactic acid [Rezaei et al 2018] and polycaprolactone [Asadian et al 2017].
Viscosity
Another important solution property to consider is its viscosity. For highly viscous solution, the electrical charges may not generate sufficient strength to stretch the solution to form fibers. If a solution is so viscous that it is almost "gel-like", it may be necessary to reduce the concentration of the solution. However, below a certain concentration, the electrospinning jet may breaks up into droplets and no fiber is formed.
In the preparation of the solution for electrospinning, it is useful to be able to predict whether the solution has sufficient viscosity to be stretched into fibers. For a polymer solution, the viscosity of the solution is determined by the chain entanglements of the polymer molecules. In a dilute solution, the distance between polymer chains are sufficiently far apart such that there is no overlap in the molecules. At a critical concentration, c*, the polymer chains start to overlap. Electrospinning at this concentration is likely to give rise to a mixture of beads and fibers. An entanglement number for the solution may be used to predict the formation of fibers in electrospinning where the entanglement number,
n
e is given by,
M
w is the molecular weight,
M
e is the solution entanglement molecular weight,
ϕ
p is the polymer volume fraction.
The study by Shenoy etal (2005) showed that when ne is more than 3.5, smooth fibers were formed for polystyrene, polylactic acid and polyethylene oxide dissolved corresponding good solvent. Below that, a mixture of beads and fibers are produced and below 2, no fibers were formed.
Since electrospinning is used to generate nanofibers, it is important to note that a higher molecular weight polymer or higher solution concentration will lead to an increase in viscosity and greater fiber diameter. As the viscosity increases, the charges that initiate the spinning may be insufficient to stretch the polymer solution to the desired fiber diameter.
For polymers, it is possible to use higher molecular weight specimens for electrospinning. However, for lower molecular weight molecules, one method is to increase the concentration of the solution to bring the solution viscosity sufficiently high for electrospinning into fibers. Cyclodextrins (CDs) are oligomers and their solution viscosity does not come from chain entanglements. Electrospinning is possible due to hydrogen bond interactions among CD aggregates at high concentrations. Topuz et al (2020) was able to get beads free fibers at solution concentration of 180 w/v % and diameters less than 400 nm with 1 wt% (with respect to CD) quaternary ammonium salt, tetraethylammonium bromide (TEAB) in the solution. While it is possible to electrospin beadless CD fibers without the addition of salt, the fiber diameter would be more than 1 micron. The addition of salt increases the conductivity of the solution such that the electrospinning CD jets can be stretched further to bring the diameter down to the nanometer dimension.
Most polymers of low molecular weight will not yield smooth fibers with increasing concentration. However, interaction between two different low molecular weight polymers may render the solution mixture electrospinnable. Perez-Puyana et al (2025) showed that with a binary polymer solution at a specific ratio of low molecular weight polycaprolactone (PCL) and gelatin, it is possible to electrospun smooth fibers although individually they are not electrospinnable. The ratio of PCL:gelatin plays an important role in optimizing chain entanglement to allow smooth fibers formation. At a PCL:gelatin ratio of 16/4 and 20/4, there is optimum viscosity and smooth fibers are electrospun. At a higher ratio of 24/4 and 32/4, beads appeared on the fibers again. The appearance of beads at higher ratios may be due to decrease in polymer miscibility and subsequent polymer phase separation.
Other effects of molecular weight and the corresponding solution viscosity on electrospinning includes the amount of secondary jet formation and deposition area. At high viscosity secondary jets may be discouraged from splitting off from the main jet [Zhao et al 2004]. A higher viscosity also tends to reduce the deposition area as it resists the charge induced bending instability [Mituppatham et al 2004].
Where two polymers were blended to form a single solution, a high difference in the viscosity for each individual polymer solution may cause significant viscosity fluctuations in their blended form during electrospinning. This in turn has been used to form electrospun membranes with bimodal fiber diameter distribution. Zhao et al (2023) tested this concept using a mixture of polystyrene (PS) and polyvinylidene fluoride-hexafluoropropylene copolymer (PVDF-HFP) with the viscosity of PVDF-HFP solution much higher than PS solution. During electrospinning, micro-phase separation from the incompatibility between PS and PVDF-HFP caused regions of low and high viscosity. At sufficiently high voltage, a finer jet from a region of low viscosity split off from the high viscosity region resulting in the finer and thicker fibers respectively. The same phenomenon was observed for incompatible polymers such as polyacrylonitrile/polyurethane (PAN/TPU) and polystyrene/polyvinylpyrrolidone (PS/PVP).
Surface tension
For the initiation of electrospinning, the charged solution needs to overcome the surface tension of the solution. Surface tension tries to reduce the surface area per unit mass of a liquid by forming spheres while the electrical charges on the electrospinning jet try to increase the surface area through elongation. While most solvents tend to have low surface tension, water has a much higher surface tension and this makes electrospinning with water more challenging as the jet is more likely to break up into droplets or the formation of beaded fibers. Replacement of water by solvent or having solvent in the solution has been shown to reduce the surface tension and favor smooth fiber formation [Fong et al 1999]. Surface tension can be reduced by using surfactants but this also introduces impurities to the electrospun product. Cationic surfactant such as hexadecyl trimethyl ammonium bromide (HTAB) not only reduces the surface tension of the solution, it also introduces additional charge carriers to the solution. This has the dual function of reducing beads formation while increasing fiber stretching to produce finer fibers. Zheng et al (2014) was able to produce polyvinylidene fluoride electrospun fibers with average diameter less than 65 nm with the addition of HTAB.
Having additives that reduce surface tension and increasing solution conductivity may also increase the spread of fibers with different diameters. Zhou et al (2022) showed that with the addition of sodium dodecyl sulfate (SDS) to polyvinylidene fluoride (PVDF) solution, fibers with smaller diameters start to branch off thicker fibers. As SDS increases the conductivity and reduces the surface tension of the solution. Greater electrification of the electrospinning jet and lower surface tension encourages side branches to erupt from the main electrospinning jet. Upon solidification, the membrane would contain a mixture of thicker and thinner fibers.
Solvent volatility
As the electrospinning jet travel towards the collector, fibers are formed when the stretched jet solidifies upon solvent evaporation. Solution prepared from solvents with very low volatility may result in wet fibers, fused fibers [Mit-uppatham et al 2004] or even no fiber collection. On the contrary, a high volatility may result in intermittent spinning due to solidification of the polymer at the spinneret tip. For a solvent mixture where one of the solvents is of high volatility, rapid vaporization of one component of the solution will also lead to the gradual formation of a polymer skin at the tip of the spinneret. This skin may grow and eventually choke off the spinning solution until more solution has been dispensed to dislodge the solidified plug. Such spinning behaviour is likely to introduce artefacts on the surface of the membrane [Knopf 2009].
With polyvinyl pyrrolidone, Yuya et al (2010) mixed the polymer with four different solvents, methanol, ethanol, water and dimethyl formamide (DMF) in the order of increasing boiling point. With a relative humidity of 30% and solution concentration of 10 wt%, solid fibers were obtained when methanol and ethanol were used as solvents. However, wet blobs and films were formed when solution from water and DMF were electrospun thus demonstrating the effect of solvent volatility on fiber formation.
Using a more volatile solvent for electrospinning has also been shown to result in ribbon/flat fibers and fibers with surface pores. Celebioglu and Uyar (2011) demonstrated this effect when they electrospin cellulose acetate with dichloromethane and acetone mixture. This combination was shown to produce ribbon fibers with surface pores. However, when the more volatile dichloromethane was replaced with less volatile N,N-dimethylacetamide, round fibers without pores were produced.
Solution phase transition
A solution phase diagram shows the interaction between the polymer and solvent in terms of its solubility at various temperatures. The phase diagram may be used to predict the conditions where the polymer will undergo phase separation and form porous structures. In an environment with high humidity, water may be absorbed into the electrospinning solution and forms a ternary phase system. Fashandi and Karimi (2012) did a comprehensive study on the effects of N, N-dimethyl formamide (DMF) and tetrahydrofuran (THF) at different ratios for electrospinning polystyrene solution across different temperature and humidity. With THF, the miscibility area is larger and only solid polystyrene fibers were formed regardless of humidity. However, DMF has a smaller miscibility area and porous (internal or surface) fibers were formed especially at higher humidity and lower temperature.
Published date: 12 June 2012
Last updated: 18 February 2025
▼ Reference
-
Asadian M, Grande S, Morent R, Nikiforov A, Declercq H, De Geyter N. Effects of pre- and post-electrospinning plasma treatments on electrospun PCL nanofibers to improve cell interactions. IOP Conf. Series: Journal of Physics: Conf. Series 2017; 841: 012018.
Open Access
-
Celebioglu A, Uyar T. Electrospun porous cellulose acetate fibers from volatile solvent mixture. Materials Letters 2011; 65: 2291.
-
Choi J S, Lee S W, Jeong L, Bae S H, Min B C, Youk J H, Park W H (2004) Effect of organosolublesalts on the nanofibrous structure of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Int. J Biological Macromol. 34 pp. 249.
-
Fashandi H, Karimi M. Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere. Polymer 2012; 53: 5832.
-
Fong H, Chun I, Reneker D H. Beaded nanofibers formed during electrospinning. Polymer 1999; 40: 4585.
-
Knopf J A. Investigation of Linear Electrospinning Jets. Bachelor Thesis 2009. University of Delaware.
-
Lin T, Wang H, Wang H, Wang X. (2004) The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 15 pp. 1375
-
Mit-uppatham C, Nithitanakul M, Pupaphol P (2004) Ultrafine Electrospun Polyamide-6 Fibers: Effect of Solution Conditions on Morphology and Average Fiber Diameter. Macromol. Chem Phys. 205 pp.2327
-
Mit-uppatham C, Nithitanakul M, Supaphol P. Effects of Solution Concentration, Emitting Electrode Polarity, Solvent Type, and Salt Addition on Electrospun Polyamide-6 Fibers: A Preliminary Report. Macromol. Symp. 2004; 216: 293.
-
Perez-Puyana V M, Romero A, Guerrero A, Moroni L, Wieringa P A. Enabling low molecular weight electrospinning through binary solutions of polymer blends. Next Materials 2025; 6: 100306.
https://www.sciencedirect.com/science/article/pii/S294982282400203X Open Access
-
Rezaei F, Nikiforov A, Morent R, De Geyter N. Plasma Modification of Poly Lactic Acid Solutions to Generate High Quality Electrospun PLA Nanofibers. Sci Rep 2018; 8: 2241.
Open Access
-
Sadaf A, Elter M, Mager D, Bunz U H F, Islam M, Korvink J G. Wall Microstructures of High Aspect Ratio Enabled by Near-Field Electrospinning. Advanced Engineering Materials 2022; 24: 2101740.
Open Access
-
Shenoy S L, Bates W D, Frisch H L, Wnek G E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific polymer-polymer interaction limit. Polymer 46; 2005: 3372.
-
Shi Q, Vitchuli N, Nowak J, Lin Z, Guo B, McCord M, Bourham M, Zhang X. Atmospheric Plasma Treatment of Pre-Electrospinning Polymer Solution:A Feasible Method to Improve Electrospinnability. Journal of Polymer Science Part B: Polymer Physics 2010; 49: 115.
-
Son W K, Youk J H, Lee T S, Park W H. (2004) Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. J. Polym. Sci. B 42 pp. 5.
-
Stanger J J. Charge Transfer Mechanism in Electrospinning. MSc Thesis. University of Canterbury 2008.
Open Access
-
Topuz F, Celebioglu A, Aytac Z, Uyar T. Influence of salt addition on polymer-free electrospinning of cyclodextrin nanofibers. Nano Ex 2020; 1: 020041.
Open Access
-
Wu Y K, Wang L, Fan J, Shou W, Zhou B M, Liu Y. Multi-Jet Electrospinning with Auxiliary Electrode: The Influence of Solution Properties. Polymers 2018; 10(6): 572.
Open Access
-
Xue N, Li X, Bertulli C, Li Z, Patharagulpong A, Sadok A, Huang Y Y S. Rapid Patterning of 1-D Collagenous Topography as an ECM Protein Fibril Platform for Image Cytometry. PLoS ONE 2014; 9(4): e93590. doi:10.1371/journal.pone.0093590.
Open Access
-
Yuya N, Kai W, Kim B S, Kim I S. Morphology controlled electrospun poly(vinyl pyrrolidone) fibers: effects of organic solvent and relative humidity. Journal of Materials Science and Engineering with Advanced Technology 2010; 2: 97.
-
Zhao S L, Wu X H, Wang L G, Huang Y (2004) Electrospinning of Ethyl-Cyanoethyl Cellulose/Tetrahydrofuran Solutions. J Appl. Polym. Sci. 91 pp. 242.
-
Zhao Y, Zhang Z, Zhang Y, Huang Y, Chen Y, Chen B, Kang W, Ju J. Fabrication of PS/PVDF-HFP Multi-Level Structured Micro/Nano Fiber Membranes by One-Step Electrospinning. Membranes. 2023; 13(10):807.
Open Access
-
Zheng J Y, Zhuang M F, Yu Z J, Zheng G F, Zhao Y, Wang H, Sun D H. The Effect of Surfactants on the Diameter and Morphology of Electrospun Ultrafine Nanofiber. Journal of Nanomaterials 2014; 687298: 9 pages.
Open Access
-
Zhong X H, Kim K S, Fang D F, Ran S F, Hsiao B S, Chu B. (2002) Structure and process relationship of Electrospun bioabsorbable nanofiber membranes. Polymer 43 pp. 4403.
-
Zhou G, Liu R, Xu Q, Wang K, Wang Y, Ramakrishna S. Dual-Structure PVDF/SDS Nanofibrous Membranes for Highly Efficient Personal Protection in Mines. Membranes. 2022; 12(5):482.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech