Electrospinning under suitable conditions, is able to produce nanofibrous nonwoven membrane with nanowebs between fibers. Such nanowebs are made out of fibers with diameters that are substantially smaller than primary electrospun fibers and often reaching 10s of nanometer or smaller. The fibers forming the nanowebs are typically fused together at the junctions. While nanowebs have been spun across different polymers, it is yet unclear on the mechanism for its formation.
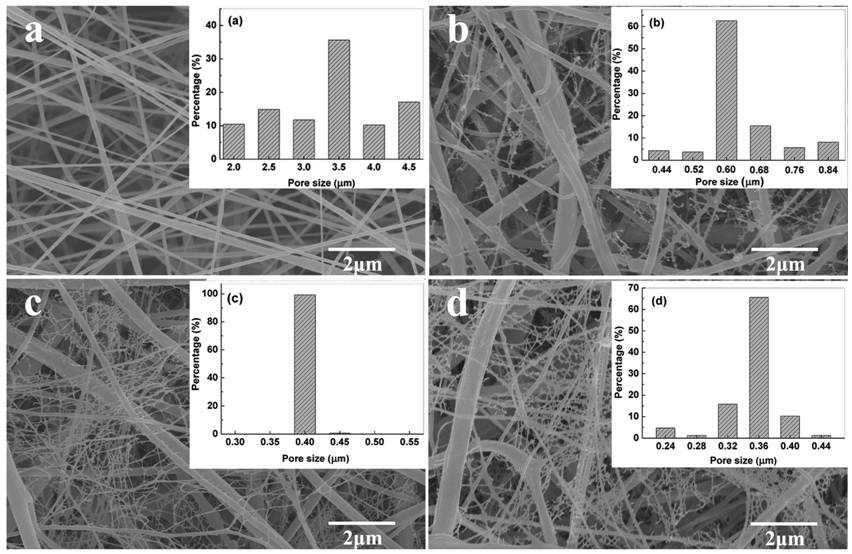
One of the earliest studies on the formation of nanoweb is carried out by Ding et al (2006). The group proposed that the nanowebs were formed by suspended charged droplets ejected from the electrospinning tip that spread out to form a thin film and subsequent phase separation caused the formation of nanowebs on the deposited electrospun fibers. Using polyacrylic acid (PAA) as their study subject, their study suggested that a higher voltage encourages the formation of nanowebs and this has been attributed to the need for a higher electrostatic force to expand and deform the charged droplets into films. Increasing concentration of PAA resulted in the formation of denser nanowebs with smaller pores between the nanoweb fibers. Distance between the electrospinning tip and the collector was also found to influence the diameter of the nanoweb fibers with shorter distance leading to larger diameter. This has been attributed to the reduced flight time for full expansion of the nanowebs.
Instead of phase separation, a different model has been proposed and this is explained by spontaneous splitting of secondary jets in more conductive solution under the electric field. The dependency of higher conductivity of the solution in the formation of nanowebs have been shown by several researchers. Barakat et al (2009) showed the influence of salt addition on Nylon-6, polyvinyl alcohol and polyurethane. Using NaCl, KBr, CaCl2 and H2PtCl6, they showed that the formation of the nanowebs were almost independent of the salts. Later work by Li et al (2016a) using high speed camera showed evidence of nanowebs formed from splitting from the primary electrospinning jet. In their experiment, with tetrabutylammonium chloride (TBAC) salt added to polyvinylidene fluoride (PVDF) solution, nanowebs were formed between the primary electrospun fibers. Massaglia et al (2017) demonstrated the formation of nanowebs in high molecular weight polyethylene oxide (PEO) with multiwall carbon nanotubes (MWCNT). Sodium polystyrene sulfonate (Na-PSS) was added to facilitate dispersion of MWCNT in the solution although it may also facilitate formation of nanowebs. Their studies showed that high molecular weight (1000 kDa) is essential for formation of nanowebs in PEO as lower molecular weight PEO (600 kDa) fails to produce nanowebs. They hypothesized that unbalanced distribution of charges causes the formation of nanowebs. With higher molecular weight, reduction of ion mobility in the solution facilitate the presence of unbalanced charge distribution. Due to jet splitting, local electric field between adjacent jets encourages spontaneous eruption of fine jets between them which later solidified to form the nanowebs. Increasing the concentration of MWCNT in the solution produces more nanowebs. Zhang et al (2017) proposed that the formation of nanoweb requires the charge to mass ratio (e/m) of the electrospinning solution to exceed the critical value of "droplet mode" and phase separation. Based on poly(m-phenylene isophthalamide) (PMIA) solution, they calculated that the theoretical e/m threshold of the fluid to be 1.19 c/kg. When the relative humidity is under 25%, the PMIA solution was 1.33 c/kg which is much higher than the threshold e/m. Nanowebs was formed at such low humidity. At higher relative humidity of 40%, no nanowebs were formed and the e/m ratio was found to be 0.077 c/kg.
The presence of nanowebs between electrospun fibers has the potential to improve the properties or widen the applications of the membrane. Pant 2015 showed that inter-fibers nanowebs from adding small amount of methoxy poly(ethylene glycol) (MPEG) oligomer to nylon-6 solution was able to increase the mechanical strength of the membrane. The improvement in mechanical strength has been attributed to the inter-connected nanowebs forming linkages between primary electrospun fibers. Li et al (2016b) demonstrated the use of such membrane for microfiltration. Using electrospun polyvinylidene fluoride (PVDF) with tetrabutylammonium chloride (TBAC) added, the resultant membrane was able to reject 99.9% of 300 nm polystyrene particles and a high pure water flux of 2.88 x 104 L.m-2h-1 under the pressure of 25 psi. With electrospun PVDF membrane without the nanowebs, the rejection was only 46%. Despite the good rejection rate, more studies are needed to determine the drop in flux as the particles accumulates and the recovery capability of this membrane.
Published date: 21 February 2017
Last updated: 26 September 2017
▼ Reference
-
Barakat N A M, Kanjwal M A, Sheikh F A, Kim H Y. Spider-net within the N6, PVA and PU electrospun nanofiber mats using salt addition: Novel strategy in the electrospinning process. Polymer 2009; 50: 4389.
-
Ding B, Li C, Miyauchi Y, Kuwaki O, Shiratori S. Formation of novel 2D polymer nanowebs
via electrospinning. Nanotechnology 2006; 17: 3685.
-
Li Z, Xu Y, Fan L, Kang W, Cheng B. Fabrication of polyvinylidene fluoride tree-like nanofiber via one-step electrospinning. Materials & Design 2016; 92: 95.
-
Li Z, Kang W, Zhao H, Hu M, Wei N, Qiu J, Cheng B. A Novel Polyvinylidene Fluoride Tree-Like Nanofiber Membrane for Microfiltration. Nanomaterials 2016b; 6: 152.
Open Access
-
Massaglia G, Chiodoni A, Salvador G P, Delmondo L, Muñoz-Tabares J A, Bocchini S, Sacco A, Bianco S, Saracco G, Quaglio M. Defining the role of nanonetting in the electrical behaviour of composite nanofiber/nets. RSC Adv 2017; 7: 38812.
Open Access
-
Pant H R. Biomimetic Spider-web like Electrospun Nanofibrous Membrane of Nylon-6 for Future Air Filtration. Journal of the Institute of Engineering 2015; 11: 108.
-
Zhang S, Liu H, Yin X, Li Z, Yu J, Ding B. Tailoring Mechanically Robust Poly(m-phenylene isophthalamide) Nanofiber/nets for Ultrathin High-Efficiency Air Filter. Scientific Reports 2017; 7: 40550.
Open Access
▲ Close list
 ElectrospinTech
ElectrospinTech